Abstract
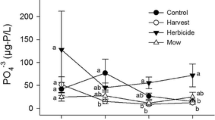
Wetland ecosystems maintain and improve water quality through the process of denitrification, an increasingly important ecosystem service due to global N pollution. Invasive plants have the potential to disrupt denitrification by altering the environmental conditions that facilitate this process. Great Lakes coastal wetlands are experiencing widespread invasion by highly productive hybrid cattail with largely uncertain biogeochemical effects. Through field and controlled mesocosm studies, we sought to determine the effects of cattail invasion through time on denitrification rates and associated environmental factors in a Great Lakes coastal wetland. In the field, we found that cattail density correlated with increased denitrification and a suite of environmental and plant community characteristics and denitrification rates were positively correlated with NH4 +, sediment organic matter, reduced water levels, and cattail stand age. Through our controlled mesocosm study, we documented conditions 1- and 5-year following invasion and found that denitrification rates and soil organic matter increased in year 5, and cattail and year-since-invasion altered plant communities and soil NH4 +. Only a weak correlation between denitrification rates and cattail treatments was noted, however, owing to high replicate variability. Our results indicate that with increasing cattail residence time, one ecosystem service, biodiversity, was negatively impacted, while two other services, denitrification and sediment carbon accumulation, were enhanced. Thus, this highly invaded wetland still provides valuable services to aquatic ecosystems and to society. A holistic perspective is therefore critical when evaluating invasive species impacts in which negative impacts are weighed against other ecosystem services, which may be stimulated.





Similar content being viewed by others
References
Albert DA, Wilcox DA, Ingram JW, Thompson TA (2005) Hydrogeomorphic classification for Great Lakes coastal wetlands. J Gt Lakes Res 31:129–146
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Angeloni NL, Jankowski KJ, Tuchman NC, Kelly JJ (2006) Effects of an invasive cattail species (Typha × glauca) on sediment nitrogen and microbial community composition in a freshwater wetland. FEMS Microbio Lett 263:86–92
APHA (2005) Standard methods for the examination of water and wastewater, vol 21. American Public Health Association, Washington, D.C
Bachand PAM, Horne AJ (1999) Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng 14:17–32
Bernot MJ, Dodds WK, Gardner WS, McCarthy MJ, Sobolev D, Tank JL (2003) Comparing denitrification estimates for a Texas estuary by using acetylene inhibition and membrane inlet mass spectrometry. Appl Environ Microb 69:5950–5956
Bivand R, Pebesma EJ, Gómez-Rubio V (2008) Applied spatial data analysis with R. Springer, New York
Boers AM, Zedler JB (2008) Stabilized water levels and Typha invasiveness. Wetlands 28(3):676–685
Boers AM, Veltman RLD, Zedler JB (2007) Typha × glauca dominance and extended hydroperiod constrain restoration of wetland diversity. Ecol Eng 29:232–244
Bruesewitz DA, Tank JL, Bernot MJ, Richardson WB, Strauss EA (2006) Seasonal effects of the zebra mussel (Dreissena polymorpha) on sediment denitrification rates in Pool 8 of the Upper Mississippi River. Can J Fish Aquat Sci 63:957–969
Camargo JA, Alonso Á (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32:831–849
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Dahnke WC (1990) Testing soils for available nitrogen. In: Westerman RL (ed) Soil testing and plant analysis. ASA, Madison, pp 120–140
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Farrer EC, Goldberg DE (2009) Litter drives ecosystem and plant community changes in cattail invasion. Ecol Appl 19:398–412
Findlay SEG, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625
Freyman MJ (2008) The effect of litter accumulation of the invasive cattail Typha × glauca on a Great Lakes coastal marsh. Thesis, Loyola University Chicago
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Groffman P, Holland E, Myrold D, Robertson G, Zou X (1999) Denitrification. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 272–288
Groffman PM, Altabet MA, Böhlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Gutierrez JL, Jones CG (2006) Physical ecosystem engineers as agents of biogeochemical heterogeneity. Bioscience 56:227–236
Hernandez ME, Mitsch WJ (2007) Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands. J Environ Qual 36:333–342
Jain AK, Briegleb BP, Minschwaner K, Wuebbles DJ (2000) Radiative forcings and global warming potentials of 39 greenhouse gases. J Geophys Res 105:20773–20790
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass-spectrometer for rapid high-precision determination of N-2, O-2, and Ar in environmental water samples. Anal Chem 66:4166–4170
Keddy P (2000) Wetland ecology: principles and conservation. Cambridge University Press, Cambridge
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Larkin DJ, Freyman MJ, Lishawa SC, Geddes P, Tuchman NC (2012a) Mechanisms of dominance by the invasive hybrid cattail Typha × glauca. Biol Invasions 14:65–77
Larkin DJ, Lishawa SC, Tuchman NC (2012b) Appropriation of nitrogen by the invasive cattail Typha × glauca. Aquat Bot 100:62–66
Lin YF, Jing SR, Wang TW, Lee DY (2002) Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ Pollut 119:413–420
Lishawa SC, Albert DA, Tuchman NC (2010) Water level decline promotes Typha × glauca establishment and vegetation change in Great Lakes coastal wetlands. Wetlands 30:1085–1096
Lishawa SC, Treering DJ, Vail LM, McKenna O, Grimm EC, Tuchman NC (2013) Reconstructing plant invasions using historical aerial imagery and pollen core analysis: Typha in the Laurentian Great Lakes. Divers Distrib 19:14–28
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Marchante E, Kjøller A, Struwe S, Freitas H (2008) Short-and long-term impacts of Acacia longifolia invasion on the belowground processes of a Mediterranean coastal dune ecosystem. Appl Soil Ecol 40:210–217
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Mitchell ME, Lishawa SC, Geddes P, Larkin DJ, Treering D, Tuchman NC (2011) Time-dependent impacts of cattail invasion in a Great Lakes coastal wetland complex. Wetlands 31:1143–1149
Murray RE, Knowles R (1999) Chloramphenicol inhibition of denitrifying enzyme activity in two agricultural soils. Appl Environ Microb 65:3487–3492
Myrold D, Tiedje J (1985) Establishment of denitrification capacity in soil: effects of carbon, nitrate and moisture. Soil Biol Biochem 17:819–822
Oksanen J (2006) Multivariate analysis of ecological communities in R: vegan tutorial. http://ocw.um.es/ciencias/geobotanica/otros-recursos-1/documentos/vegantutorial.pdf. Accessed 13 January 2014
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHN, Wagner H (2012) Vegan Community Ecology Package. R package version 2.0-4. http://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 13 May 2013
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Pfenning KS, McMahon PB (1997) Effect of nitrate, organic carbon, and temperature on potential denitrification rates in nitrate-rich riverbed sediments. J Hydrol 187:283–295
R Development Core Team (2009) R: a language and environment for statistical computing. R Version 2.12.1. R Foundation for Statistical Computing, Vienna
Rooth JE, Stevenson JC, Cornwall JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26:475–483
Song K, Hojeong K, Zhang L, Mitsch WJ (2012) Seasonal and spatial variations of denitrification and denitrifying bacterial community structure in created riverine wetlands. Ecol Eng 38:130–134
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Strong DT, Fillery IRP (2002) Denitrification response to nitrate concentrations in sandy soils. Soil Biol Biochem 34:945–954
Tuchman NC, Jankowski KJ, Geddes P, Wildova R, Larkin DJ, Goldberg DE (2009) Patterns of environmental change associates with Typha × glauca invasion in a Great Lakes coastal wetland. Wetlands 29:964–975
Tulbure MG, Johnston CA (2010) Environmental conditions promoting non-native Phragmites australis expansion in Great Lakes coastal wetlands. Wetlands 30:577–587
Tulbure MG, Johnston CA, Auger DL (2007) Rapid invasion of a Great Lakes coastal wetland by non-native Phragmites australis and Typha. J Gt Lakes Res 33:269–279
Vaccaro LE, Bedford BL, Johnston CA (2009) Litter accumulation promotes dominance of invasive species of cattails (Typha spp.) in Lake Ontario wetlands. Wetlands 29:1036–1048
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wafer CC, Richards JB, Osmond DL (2004) Construction of platinum-tipped redox probes for determining soil redox potential. J Environ Qual 33:2375–2379
Wilcox DA, Apfelbaum SI, Hiebert RD (1985) Cattail invasion of sedge meadows following hydrologic disturbance in the Cowles Bog wetland complex, Indiana Dunes National Lakeshore. Wetlands 4:115–128
Wilcox DA, Kowalski KP, Hoare HL, Carlson ML, Morgan HN (2008) Cattail invasion of sedge/grass meadows in Lake Ontario: photointerpretation analysis of sixteen wetlands over five decades. J Gt Lakes Res 34:301–323
Windham L, Ehrenfeld JG (2003) Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecol Appl 13:883–896
Windham L, Meyerson LA (2003) Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes of the northeastern US. Estuaries 26:452–464
Witkowski ETF, Wilson M (2001) Changes in density, biomass, seed production and soil seed banks of the non-native invasive plant, Chromolaena odorata, along a 15 year chronosequence. Plant Ecol 152:13–27
Woo I, Zedler JB (2002) Can nutrients alone shift a sedge meadow towards dominance by the invasive Typha × glauca? Wetlands 22:509–521
Yoshinari T, Knowles R (1976) Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun 69:705–710
Zedler JB (2003) Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ 1:65–72
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452
Acknowledgments
We thank Michael Grant for assistance in chemical analysis, Steve Bertman and Jennifer Tank for analytical assistance and method development, and Chester Elliot, Emily Kay, Erica Mynarich, Brian Schuetz, and Sharon Shattuck for many hours of work in the lab and field. This research was supported by National Science Foundation grant DGE-0343372 to N. Tuchman and M. Freyman, National Science Foundation REU Site: Biosphere–Atmosphere Interactions in a Changing Global Environment, Award 0851421 to K. Nadelhoffer, D. Karowe, and M.A. Carroll, and by an award from the Loyola University Chicago Office of the Provost to N. Tuchman.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lishawa, S.C., Jankowski, K., Geddes, P. et al. Denitrification in a Laurentian Great Lakes coastal wetland invaded by hybrid cattail (Typha × glauca). Aquat Sci 76, 483–495 (2014). https://doi.org/10.1007/s00027-014-0348-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-014-0348-5