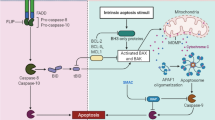
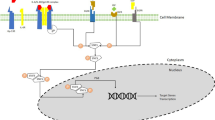
Abstract
Breast cancer (BC) is one of the most common cancers in females and is responsible for the highest cancer-related deaths following lung cancer. The complex tumor microenvironment and the aggressive behavior, heterogenous nature, high proliferation rate, and ability to resist treatment are the most well-known features of BC. Accordingly, it is critical to find an effective therapeutic agent to overcome these deleterious features of BC. Resveratrol (RES) is a polyphenol and can be found in common foods, such as pistachios, peanuts, bilberries, blueberries, and grapes. It has been used as a therapeutic agent for various diseases, such as diabetes, cardiovascular diseases, inflammation, and cancer. The anticancer mechanisms of RES in regard to breast cancer include the inhibition of cell proliferation, and reduction of cell viability, invasion, and metastasis. In addition, the synergistic effects of RES in combination with other chemotherapeutic agents, such as docetaxel, paclitaxel, cisplatin, and/or doxorubicin may contribute to enhancing the anticancer properties of RES on BC cells. Although, it demonstrates promising therapeutic features, the low water solubility of RES limits its use, suggesting the use of delivery systems to improve its bioavailability. Several types of nano drug delivery systems have therefore been introduced as good candidates for RES delivery. Due to RES’s promising potential as a chemopreventive and chemotherapeutic agent for BC, this review aims to explore the anticancer mechanisms of RES using the most up to date research and addresses the effects of using nanomaterials as delivery systems to improve the anticancer properties of RES.
Graphical abstract





Similar content being viewed by others
Availability of data and material
Not applicable.
References
Goff SL, Danforth DNJCBC (2021) The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer 21:e63–e73. https://doi.org/10.1016/j.clbc.2020.06.011
Chong QY, Kok ZH, Bui NL, Xiang X, Wong AL, Yong WP, Sethi G, Lobie PE, Wang L, Goh BC (2020) A unique CDK4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacol Res 156:104686. https://doi.org/10.1016/j.phrs.2020.104686
Loo SY, Syn NL, Koh AP, Teng JC, Deivasigamani A, Tan TZ, Thike AA, Vali S, Kapoor S, Wang X, Wang JW (2021) Epigenetic derepression converts PPARγ into a druggable target in triple-negative and endocrine-resistant breast cancers. Cell Death Discov 7(1):1–15. https://doi.org/10.1038/s41420-021-00635-5
Thakur KK, Kumar A, Banik K, Verma E, Khatoon E, Harsha C, Sethi G, Gupta SC, Kunnumakkara AB (2021) Long noncoding RNAs in triple-negative breast cancer: a new frontier in the regulation of tumorigenesis. J Cell Physiol 236(12):7938–7965. https://doi.org/10.1002/jcp.30463
Jia LY, Shanmugam MK, Sethi G, Bishayee A (2016) Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anticancer Drugs 27:147–155. https://doi.org/10.1097/CAD.0000000000000328
Mohan CD, Srinivasa V, Rangappa S, Mervin L, Mohan S, Paricharak S, Baday S, Li F, Shanmugam MK, Chinnathambi A, Zayed ME (2016) Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE 11:e0153155. https://doi.org/10.1371/journal.pone.0153155
Lu W, Ni Z, Jiang S, Tong M, Zhang J, Zhao J, Feng C, Jia Q, Wang J, Yao T, Ning H (2021) Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway. Phytother Res 35:1495–1507. https://doi.org/10.1002/ptr.6915
El Ansari R, McIntyre A, Craze ML, Ellis IO, Rakha EA, Green ARJH (2018) Altered glutamine metabolism in breast cancer; subtype dependencies and alternative adaptations. Histopathology 72:183–190. https://doi.org/10.1111/his.13334
Cappelletti V, Iorio E, Miodini P, Silvestri M, Dugo M, Daidone MGJD (2017) Metabolic footprints and molecular subtypes in breast cancer. Dis Mark 2017:8687851. https://doi.org/10.1155/2017/7687851
Chen L, Yuan Y, Kar S, Kanchi MM, Arora S, Kim JE, Koh PF, Yousef E, Samy RP, Shanmugam MK, Tan TZ (2017) PPARγ ligand-induced annexin A1 expression determines chemotherapy response via deubiquitination of death domain kinase RIP in triple-negative breast cancers. Mol Cancer Ther 16:2528–2542. https://doi.org/10.1158/1535-7163.MCT-16-0739
Liu L, Ahn KS, Shanmugam MK, Wang H, Shen H, Arfuso F, Chinnathambi A, Alharbi SA, Chang Y, Sethi G, Tang FR (2019) Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor-negative breast cancer cells. J Cell Biochem 120:4504–4513. https://doi.org/10.1002/jcb.27738
Shen Q, Reedijk M (2021) Notch signaling and the breast cancer microenvironment. In: Notch signaling in embryology and cancer. Springer, pp 183–200. https://doi.org/10.1007/978-3-030-55031-8_12
Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q et al (2017) Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 8:27990–27996. https://doi.org/10.18632/oncotarget.15856
Koual M, Tomkiewicz C, Cano-Sancho G, Antignac J-P, Bats A-S, Coumoul XJEH (2020) Environmental chemicals, breast cancer progression and drug resistance. Environ Health 19:1–25
Bhuvanalakshmi G, Basappa RKS, Dharmarajan A, Sethi G, Kumar AP et al (2017) Breast cancer stem-like cells are inhibited by diosgenin, a steroidal saponin, by the attenuation of the Wnt β-catenin signaling via the Wnt antagonist secreted frizzled related protein-4. Front Pharmacol 8:124. https://doi.org/10.3389/fphar.2017.00124
Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M (2020) Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther 12:211–229. https://doi.org/10.2147/BCTT.S270799
Wang C, Kar S, Lai X, Cai W, Arfuso F, Sethi G et al (2018) Triple negative breast cancer in Asia: an insider’s view. Cancer Treat Rev 62:29–38. https://doi.org/10.1016/j.ctrv.2017.10.014
Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G et al (2000) HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol 18:3471–3479. https://doi.org/10.1200/JCO.2000.18.20.3471
Parisot JP, Hu XF, DeLuise M, Zalcberg JR (1999) Altered expression of the IGF-1 receptor in a tamoxifen-resistant human breast cancer cell line. Br J Cancer 79:693–700. https://doi.org/10.1038/sj.bjc.6690112
Tai W, Mahato R, Cheng K (2010) The role of HER2 in cancer therapy and targeted drug delivery. J Control Release 146:264–275. https://doi.org/10.1016/j.jconrel.2010.04.009
Roscigno G, Scognamiglio I, Ingenito F, Chianese RV, Palma F, Chan A et al (2020) Modulating the crosstalk between the tumor and the microenvironment using SiRNA: a flexible strategy for breast cancer treatment. Cancers (Basel) 12(12):3744. https://doi.org/10.3390/cancers12123744
Rostami N, Nikkhoo A, Khazaei-Poul Y, Farhadi S, Sadat Haeri M, MoghadaszadehArdebili S et al (2020) Coinhibition of S1PR1 and GP130 by siRNA-loaded alginate-conjugated trimethyl chitosan nanoparticles robustly blocks development of cancer cells. J Cell Physiol 235:9702–9717. https://doi.org/10.1002/jcp.29781
Xu X, Li L, Li X, Tao D, Zhang P, Gong J (2020) Aptamer-protamine-siRNA nanoparticles in targeted therapy of ErbB3 positive breast cancer cells. Int J Pharm 590:119963. https://doi.org/10.1016/j.ijpharm.2020.119963
Singh V, Kumar K, Purohit D, Verma R, Pandey P, Bhatia S et al (2021) Exploration of therapeutic applicability and different signaling mechanism of various phytopharmacological agents for treatment of breast cancer. Biomed Pharmacother 139:111584. https://doi.org/10.1016/j.biopha.2021.111584
Wu T-N, Chen H-M, Shyur L-F (2021) Current advancements of plant-derived agents for triple-negative breast cancer therapy through deregulating cancer cell functions and reprogramming tumor microenvironment. Int J Mol Sci 22(24):13571. https://doi.org/10.3390/ijms222413571
Yap KM, Sekar M, Seow LJ, Gan SH, Bonam SR, Rani NNIM et al (2021) Mangifera indica (Mango): a promising medicinal plant for breast cancer therapy and understanding its potential mechanisms of action. Breast Cancer Targets Ther 13:471–503. https://doi.org/10.2147/BCTT.S316667
Cole T, Inglis A, Nagashima M, Schreiber G (1985) Major acute-phase alpha(1)protein in the rat: structure, molecular cloning, and regulation of mRNA levels. Biochem Biophys Res Commun 126(2):719–724. https://doi.org/10.1016/0006-291X(85)90244-X
Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM et al (2019) Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci 76:1947–1966. https://doi.org/10.1007/s00018-019-03053-0
Patel SM, Nagulapalli Venkata KC, Bhattacharyya P, Sethi G, Bishayee A (2016) Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin Cancer Biol 40–41:100–115. https://doi.org/10.1016/j.semcancer.2016.03.002
Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S et al (2021) Natural product-based nanoformulations for cancer therapy: opportunities and challenges. Semin Cancer Biol 69:5–23. https://doi.org/10.1016/j.semcancer.2019.08.014
Li F, Shanmugam MK, Chen L, Chatterjee S, Basha J, Kumar AP et al (2013) Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev Res (Phila) 6:843–854. https://doi.org/10.1158/1940-6207.CAPR-13-0070
Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP et al (2018) Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients 10(5):645. https://doi.org/10.3390/nu10050645
Bishayee A, Ahmed S, Brankov N, Perloff M (2011) Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci 16:980–996. https://doi.org/10.2741/3730
Ferraz da Costa DC, Pereira Rangel L, Quarti J, Santos RA, Silva JL, Fialho E (2020) Bioactive compounds and metabolites from grapes and red wine in breast cancer chemoprevention and therapy. Molecules 25(15):3531. https://doi.org/10.3390/molecules25153531
Marcum JA (2020) Nutrigenetics/nutrigenomics, personalized nutrition, and precision healthcare. Curr Nutr Rep 9:338–345. https://doi.org/10.1007/s13668-020-00327-z
Abadi AJ, Mirzaei S, Mahabady MK, Hashemi F, Zabolian A, Hashemi F et al (2021) Curcumin and its derivatives in cancer therapy: potentiating antitumor activity of cisplatin and reducing side effects. Phytother Res 36(1):189–213. https://doi.org/10.1002/ptr.7305
Ashrafizadeh M, Najafi M, Makvandi P, Zarrabi A, Farkhondeh T, SamarghandianSJJoCP, (2020) Versatile role of curcumin and its derivatives in lung cancer therapy. J Cell Physiol 235(12):9241–9268. https://doi.org/10.1002/jcp.29819
Ashrafizadeh M, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Bagherian M et al (2020) Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: enhancing antitumor activity. Pharmaceutics 12(11):1084. https://doi.org/10.3390/pharmaceutics12111084
Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Hashemi F et al (2020) Toward regulatory effects of curcumin on transforming growth factor-beta across different diseases: a review. Front Pharmacol 11:1785. https://doi.org/10.3389/fphar.2020.585413
Ashrafizadeh M, Zarrabi A, Mirzaei S, Hashemi F, Samarghandian S, Zabolian A et al (2021) Gallic acid for cancer therapy: molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem Toxicol 157:112576. https://doi.org/10.1016/j.fct.2021.112576
Ashrafizadeh M, Zarrabi A, Orouei S, Saberifar S, Salami S, Hushmandi K et al (2021) Recent advances and future directions in anti-tumor activity of cryptotanshinone: a mechanistic review. Phytother Res 35(1):155–179. https://doi.org/10.1002/ptr.6815
Farkhondeh T, Ashrafizadeh M, Azimi-Nezhad M, Samini F, Aschenr M, Samarghandian SJCMP (2021) Curcumin efficacy in a serum/glucose deprivation-induced neuronal PC12 injury model. Curr Mol Pharmacol 14(6):1146–1155. https://doi.org/10.2174/1874467214666210203211312
Hussain Y, Mirzaei S, Ashrafizadeh M, Zarrabi A, Hushmandi K, Khan H et al (2021) Quercetin and its nano-scale delivery systems in prostate cancer therapy: paving the way for cancer elimination and reversing chemoresistance. Cancers 13(7):1602. https://doi.org/10.3390/cancers13071602
Khwairakpam AD, Bordoloi D, Thakur KK, Monisha J, Arfuso F, Sethi G et al (2018) Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol Res 133:53–64. https://doi.org/10.1016/j.phrs.2018.04.021
Mirzaei S, Gholami MH, Zabolian A, Saleki H, Farahani MV, Hamzehlou S et al (2021) Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res 171:105759. https://doi.org/10.1016/j.phrs.2021.105759
MoballeghNasery M, Abadi B, Poormoghadam D, Zarrabi A, Keyhanvar P, Khanbabaei H et al (2020) Curcumin delivery mediated by bio-based nanoparticles: a review. Molecules 25(3):689. https://doi.org/10.3390/molecules25030689
Pourbagher-Shahri AM, Farkhondeh T, Ashrafizadeh M, Talebi M, Samargahndian SJB (2021) Curcumin and cardiovascular diseases: focus on cellular targets and cascades. Pharmacotherapy 136:111214. https://doi.org/10.1016/j.biopha.2020.111214
Roy NK, Deka A, Bordoloi D, Mishra S, Kumar AP, Sethi G et al (2016) The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett 377:74–86. https://doi.org/10.1016/j.canlet.2016.04.017
Ren B, Kwah MX-Y, Liu C, Ma Z, Shanmugam MK, Ding L et al (2021) Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett 515:63–72. https://doi.org/10.1016/j.canlet.2021.05.001
Bishayee A (2009) Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res 2:409–418. https://doi.org/10.1158/1940-6207.CAPR-08-0160
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP et al (2017) The role of resveratrol in cancer therapy. Int J Mol Sci 18(12):2589. https://doi.org/10.3390/ijms18122589
Poschner S, Maier-Salamon A, Thalhammer T, Jäger W (2019) Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J Steroid Biochem Mol Biol 190:11–18. https://doi.org/10.1016/j.jsbmb.2019.03.001
Sinha D, Sarkar N, Biswas J, Bishayee A (2016) Resveratrol for breast cancer prevention and therapy: preclinical evidence and molecular mechanisms. Semin Cancer Biol 40–41:209–232
Delmas D, Cornebise C, Courtaut F, Xiao J, Aires VJIJoMS (2021) New highlights of resveratrol: a review of properties against ocular diseases. Int J Mol Sci 22(3):1295. https://doi.org/10.3390/ijms22031295
Takaoka MJJFSHIU (1940) Of the phenolic substrate of hellebore (Veratrum grandiflorum Loes. fil.). J Fac Sci Hokkaido Imper Univ 3:1–16
Mongioì LM, La Vignera S, Cannarella R, Cimino L, Compagnone M, Condorelli RA et al (2021) The role of resveratrol administration in human obesity. Int J Mol Sci 22(9):4362. https://doi.org/10.3390/ijms22094362
Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S (2020) Resveratrol targeting the Wnt signaling pathway: a focus on therapeutic activities. J Cell Physiol 235(5):4135–4145. https://doi.org/10.1002/jcp.29327
Riccio BV, Spósito L, Carvalho GC, Ferrari PC, Chorilli M (2020) Resveratrol isoforms and conjugates: a review from biosynthesis in plants to elimination from the human body. Arch Pharm 353(12):2000146. https://doi.org/10.1002/ardp.202000146
Lançon A, Delmas D, Osman H, Thénot JP, Jannin B, Latruffe N (2004) Human hepatic cell uptake of resveratrol: involvement of both passive diffusion and carrier-mediated process. Biochem Biophys Res Commun 316:1132–1137. https://doi.org/10.1016/j.bbrc.2004.02.164
Gowd V, Karim N, Shishir MRI, Xie L, Chen WJTiFS, Technology (2019) Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci Technol 93:81–93. https://doi.org/10.1016/j.tifs.2019.09.005
Walle T (2011) Bioavailability of resveratrol. Ann N Y Acad Sci 1215:9–15. https://doi.org/10.1111/j.1749-6632.2010.05842.x
Amini P, Nodooshan SJ, Ashrafizadeh M, Eftekhari SM, Aryafar T, Khalafi L, Musa AE, Mahdavi SR, Najafi M, Farhood B (2021) Resveratrol induces apoptosis and attenuates proliferation of MCF-7 cells in combination with radiation and hyperthermia. Curr Mol Med 21(2):142–150. https://doi.org/10.2174/1566524020666200521080953
Ashrafizadeh M, Mohammadinejad R, Farkhondeh T, Samarghandian S (2021) Protective effect of resveratrol against glioblastoma: a review. Anti-Cancer Agents Med Chem 21(10):1216–1227. https://doi.org/10.2174/1871520620666200929151139
Ashrafizadeh M, Najafi M, Orouei S, Zabolian A, Saleki H, Azami N, Sharifi N, Hushmandi K, Zarrabi A, Ahn KS (2020) Resveratrol modulates transforming growth factor-beta (tgf-β) signaling pathway for disease therapy: a new insight into its pharmacological activities. Biomedicines 8(8):261. https://doi.org/10.3390/biomedicines8080261
Ashrafizadeh M, Rafiei H, Mohammadinejad R, Farkhondeh T, Samarghandian SJCCI (2022) Anti-tumor activity of resveratrol against gastric cancer: a review of recent advances with an emphasis on molecular pathways. Cancer Cell Int 21(1):1–10. https://doi.org/10.1186/s12935-021-01773-7
Ashrafizadeh M, Taeb S, Haghi-Aminjan H, Afrashi S, Moloudi K, Musa AE et al (2020) Resveratrol as an enhancer of apoptosis in cancer: a mechanistic review. Anti-Cancer Agents Med Chem 21(17):2327–2336. https://doi.org/10.2174/1871520620666201020160348
Ashrafizadeh M, Zarrabi A, Najafi M, Samarghandian S, Mohammadinejad R, Ahn KSJPR (2020) Resveratrol targeting tau proteins, amyloid-beta aggregations, and their adverse effects: an updated review. Phytother Res 34(11):2867–2888. https://doi.org/10.1002/ptr.6732
Deng S, Shanmugam MK, Kumar AP, Yap CT, Sethi G, Bishayee A (2019) Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 125:1228–1246. https://doi.org/10.1002/cncr.31978
Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F (2017) Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol 15:503–519. https://doi.org/10.2174/1570161115666170713094319
Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG et al (2018) Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res 128:366–375. https://doi.org/10.1016/j.phrs.2017.09.014
Heimesaat MM, Mousavi S, Escher U, Lobo de Sá FD, Peh E, Schulzke J-D et al (2020) Resveratrol alleviates acute Campylobacter jejuni induced enterocolitis in a preclinical murine intervention study. Microorganisms 8(12):1858. https://doi.org/10.3390/microorganisms8121858
Ye M, Wu H, Li S (2021) Resveratrol alleviates oxygen/glucose deprivation/reoxygenation-induced neuronal damage through induction of mitophagy. Mol Med Rep 23(1):1. https://doi.org/10.3892/mmr.2020.11711
Ahmadi Z, Ashrafizadeh MJF, Pharmacology C (2020) Melatonin as a potential modulator of Nrf2. Fundam Clin Pharmacol 34(1):11–19. https://doi.org/10.1111/fcp.12498
Ashrafizadeh M, Fekri HS, Ahmadi Z, Farkhondeh T, Samarghandian SJJ (2020) Therapeutic and biological activities of berberine: the involvement of Nrf2 signaling pathway. J Cell Biochem 121:1575–1585. https://doi.org/10.1002/jcb.29392
Mirzaei S, Mohammadi AT, Gholami MH, Hashemi F, Zarrabi A, Zabolian A et al (2021) Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol Res 167:105575. https://doi.org/10.1016/j.phrs.2021.105575
Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Azami N et al (2021) Nrf2 signaling pathway in chemoprotection and doxorubicin resistance: potential application in drug discovery. Antioxidants 10(3):349. https://doi.org/10.3390/antiox10030349
Zhou Y, Lan R, Xu Y, Zhou Y, Lin X, Miao J (2020) Resveratrol alleviates oxidative stress caused by Streptococcus uberis infection via activating the Nrf2 signaling pathway. Int Immunopharmacol 89:107076. https://doi.org/10.1016/j.intimp.2020.107076
Fernández-Rodríguez JA, Almonte-Becerril M, Ramil-Gómez O, Hermida-Carballo L, Viñas-Diz S, Vela-Anero Á et al (2021) Autophagy activation by resveratrol reduces severity of experimental rheumatoid arthritis. Mol Nutr Food Res 65:e2000377. https://doi.org/10.1002/mnfr.202000377
Virk P, Al-Mukhaizeem NAR, Bin Morebah SH, Fouad D, Elobeid M (2020) Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. Food Chem Toxicol 146:111840. https://doi.org/10.1016/j.fct.2020.111840
Chang WS, Tsai CW, Yang JS, Hsu YM, Shih LC, Chiu HY et al (2021) Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J Food Biochem 45:e13666. https://doi.org/10.1111/jfbc.13666
Bostan M, Mihaila M, Petrica-Matei GG, Radu N, Hainarosie R, Stefanescu CD et al (2021) Resveratrol modulation of apoptosis and cell cycle response to cisplatin in head and neck cancer cell lines. Int J Mol Sci 22(12):6322. https://doi.org/10.3390/ijms22126322
Gadag S, Narayan R, Nayak AS, Ardila DC, Sant S, Nayak Y et al (2021) Development and preclinical evaluation of microneedle-assisted resveratrol loaded nanostructured lipid carriers for localized delivery to breast cancer therapy. Int J Pharm 606:120877. https://doi.org/10.1016/j.ijpharm.2021.120877
Chopra H, Bibi S, Islam F, Ahmad SU, Olawale OA, Alhumaydhi FA et al (2022) Emerging trends in the delivery of resveratrol by nanostructures: applications of nanotechnology in life sciences. J Nanomater 2022:3083728. https://doi.org/10.1155/2022/3083728
Maurya L, Singh S, Shah K, Dewangan HK (2022) Dual vinorelbine bitartrate and resveratrol loaded polymeric aqueous core nanocapsules for synergistic efficacy in breast cancer. J Microencapsul. https://doi.org/10.1080/02652048.2022.2070679
Annaji M, Poudel I, Boddu SH, Arnold RD, Tiwari AK, Babu RJ (2021) Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep 4(3):e1353. https://doi.org/10.1002/cnr2.1353
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Kim SM, Lee JH, Sethi G, Kim C, Baek SH, Nam D et al (2014) Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett 354(1):153–163. https://doi.org/10.1016/j.canlet.2014.08.002
Lee JH, Kim C, Kim SH, Sethi G, Ahn KS (2015) Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett 360(2):280–293. https://doi.org/10.1016/j.canlet.2015.02.024
Mohan CD, Bharathkumar H, Bulusu KC, Pandey V, Rangappa S, Fuchs JE et al (2014) Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem 289:34296–34307. https://doi.org/10.1074/jbc.M114.601104
Neophytou CM, Trougakos IP, Erin N, Papageorgis P (2021) Apoptosis deregulation and the development of cancer multi-drug resistance. Cancer 13(17):4363. https://doi.org/10.3390/cancers13174363
Fu X, Li M, Tang C, Huang Z, Najafi MJA (2021) Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis 26(11):561–573https://doi.org/10.1007/s10495-021-01689-7
Koklesova L, Liskova A, Samec M, Buhrmann C, Samuel SM, Varghese E et al (2020) Carotenoids in cancer apoptosis—the road from bench to bedside and back. Cancers 12(9):2425. https://doi.org/10.3390/cancers12092425
Patra S, Pradhan B, Nayak R, Behera C, Panda KC, Das S et al (2021) Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: current evidences and future perspectives. Phytother Res 35(8):4194–4214. https://doi.org/10.1002/ptr.7082
Yu C, Yang B, Najafi MJB (2021) Targeting of cancer cell death mechanisms by curcumin: implications to cancer therapy. Basic Clin Pharmacol Toxicol 129(6):397–415. https://doi.org/10.1111/bcpt.13648
Liang ZJ, Wan Y, Zhu DD, Wang MX, Jiang HM, Huang DL et al (2021) Resveratrol mediates the apoptosis of triple negative breast cancer cells by reducing POLD1 expression. Front Oncol 11:569295. https://doi.org/10.3389/fonc.2021.569295
Komorowska D, Gajewska A, Hikisz P, Bartosz G, Rodacka A (2021) Comparison of the effects of resveratrol and its derivatives on the radiation response of MCF-7 breast cancer cells. Int J Mol Sci 22(17):9511. https://doi.org/10.3390/ijms22179511
Costa PSD, Ramos PS, Ferreira C, Silva JL, El-Bacha T, Fialho E (2021) Pro-oxidant effect of resveratrol on human breast cancer MCF-7 cells is associated with CK2 inhibition. Nutr Cancer. https://doi.org/10.1080/01635581.2021.1977834
Wu H, Chen L, Zhu F, Han X, Sun L, Chen K (2019) The Cytotoxicity effect of resveratrol: cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins (Basel) 11(12):731. https://doi.org/10.3390/toxins11120731
Venkatadri R, Iyer AKV, Kaushik V, Azad N (2017) A novel resveratrol-salinomycin combination sensitizes ER-positive breast cancer cells to apoptosis. Pharmacol Rep 69(4):788–797. https://doi.org/10.1016/j.pharep.2017.03.024
Mirzapur P, Khazaei MR, Moradi MT, Khazaei M (2018) Apoptosis induction in human breast cancer cell lines by synergic effect of raloxifene and resveratrol through increasing proapoptotic genes. Life Sci 205:45–53. https://doi.org/10.1016/j.lfs.2018.04.035
Hernandez-Valencia J, Garcia-Villa E, Arenas-Hernandez A, Garcia-Mena J, Diaz-Chavez J, Gariglio P (2018) Induction of p53 phosphorylation at serine 20 by resveratrol is required to activate p53 target genes, restoring apoptosis in MCF-7 cells resistant to cisplatin. Nutrients 10(9):1148. https://doi.org/10.3390/nu10091148
Mondal A, Bennett LL (2016) Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed Pharmacother 84:1906–1914. https://doi.org/10.1016/j.biopha.2016.10.096
Bordoloi D, Banik K, Shabnam B, Padmavathi G, Monisha J, Arfuso F et al (2018) TIPE family of proteins and its implications in different chronic diseases. Int J Mol Sci 19(10):2974. https://doi.org/10.3390/ijms19102974
Patra S, Mishra SR, Behera BP, Mahapatra KK, Panigrahi DP, Bhol CS et al (2020) Autophagy-modulating phytochemicals in cancer therapeutics: current evidences and future perspectives. Semin Cancer Biol 80:205–217. https://doi.org/10.1016/j.semcancer.2020.05.008
Praharaj PP, Naik PP, Panigrahi DP, Bhol CS, Mahapatra KK, Patra S et al (2019) Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: its implication in cancer therapeutics. Cell Mol Life Sci 76:1641–1652. https://doi.org/10.1007/s00018-018-2990-x
Hao M, Yeo SK, Turner K, Harold A, Yang Y, Zhang X et al (2021) Autophagy blockade limits HER2+ breast cancer tumorigenesis by perturbing HER2 trafficking and promoting release via small extracellular vesicles. Dev Cell 56:341-355.e5. https://doi.org/10.1016/j.devcel.2020.12.016
Maiti A, Hait NC (2021) Autophagy-mediated tumor cell survival and progression of breast cancer metastasis to the brain. J Cancer 12:954–964. https://doi.org/10.7150/jca.50137
Li P, Cao G, Huang Y, Wu W, Chen B, Wang Z et al (2020) siMTA1-Loaded exosomes enhanced chemotherapeutic effect of gemcitabine in luminal-b type breast cancer by inhibition of EMT/HIF-α and autophagy pathways. Front Oncol 10:541262. https://doi.org/10.3389/fonc.2020.541262
Li QW, Zhang GL, Hao CX, Ma YF, Sun X, Zhang Y et al (2021) SANT, a novel Chinese herbal monomer combination, decreasing tumor growth and angiogenesis via modulating autophagy in heparanase overexpressed triple-negative breast cancer. J Ethnopharmacol 266:113430. https://doi.org/10.1016/j.jep.2020.113430
Swetha KL, Sharma S, Chowdhury R, Roy A (2020) Disulfiram potentiates docetaxel cytotoxicity in breast cancer cells through enhanced ROS and autophagy. Pharmacol Rep 72:1749–1765. https://doi.org/10.1007/s43440-020-00122-1
Din SRU, Zhong M, Nisar MA, Saleem MZ, Hussain A, Khinsar KH et al (2020) Latcripin-7A, derivative of Lentinula edodes C(91–3), reduces migration and induces apoptosis, autophagy, and cell cycle arrest at G(1) phase in breast cancer cells. Appl Microbiol Biotechnol 104:10165–10179. https://doi.org/10.1007/s00253-020-10918-z
Mitra D, Vega-Rubin-de-Celis S, Royla N, Bernhardt S, Wilhelm H, Tarade N et al (2021) Abrogating GPT2 in triple-negative breast cancer inhibits tumor growth and promotes autophagy. Int J Cancer 148:1993–2009. https://doi.org/10.1002/ijc.33456
Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian SJCCI (2020) Autophagy regulation using luteolin: new insight into its anti-tumor activity. Cancer Cell Int 20(1):1–9. https://doi.org/10.1186/s12935-020-01634-9
Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian SJJ (2020) Modulatory effects of statins on the autophagy: a therapeutic perspective. J Cell Physiol 235(4):3157–3168. https://doi.org/10.1002/jcp.29227
Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian SJPR (2020) Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res 34(5):911–923. https://doi.org/10.1002/ptr.6577
Ashrafizadeh M, Zarrabi A, Orouei S, Hushmandi K, Hakimi A, Zabolian A et al (2021) MicroRNA-mediated autophagy regulation in cancer therapy: the role in chemoresistance/chemosensitivity. Eur J Pharmacol 892:173660. https://doi.org/10.1016/j.ejphar.2020.173660
Vargas JE, Puga R, Lenz G, Trindade C, Filippi-Chiela E (2020) Cellular mechanisms triggered by the cotreatment of resveratrol and doxorubicin in breast cancer: a translational in vitro-in silico model. Oxid Med Cell Longev 2020:5432651. https://doi.org/10.1155/2020/5432651
Rai G, Mishra S, Suman S, Shukla Y (2016) Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: a mechanistic insight. Phytomedicine 23:233–242. https://doi.org/10.1016/j.phymed.2015.12.020
Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK (2015) The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem 116:450–457. https://doi.org/10.1002/jcb.24997
Rai G, Suman S, Mishra S, Shukla Y (2017) Evaluation of growth inhibitory response of resveratrol and salinomycin combinations against triple negative breast cancer cells. Biomed Pharmacother 89:1142–1151. https://doi.org/10.1016/j.biopha.2017.02.110
Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R (2008) Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ 15:1318–1329. https://doi.org/10.1038/cdd.2008.51
Li Q, Zan L (2022) Knockdown of ATG4A inhibits breast cancer progression and promotes tamoxifen chemosensitivity by suppressing autophagy. Mol Med Rep 25(3):1–9. https://doi.org/10.3892/mmr.2022.12617
Zhou N, Liu Q, Wang X, He L, Zhang T, Zhou H, Zhu X, Zhou T, Deng G, Qiu C (2022) The combination of hydroxychloroquine and 2-deoxyglucose enhances apoptosis in breast cancer cells by blocking protective autophagy and sustaining endoplasmic reticulum stress. Cell Death Discov 8(1):1–10. https://doi.org/10.1038/s41420-022-01074-6
Cheng T, Wang C, Lu Q, Cao Y, Yu W, Li W, Liu B, Gao X, Lü J, Pan X (2022) Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic Biol Med 180:108–120. https://doi.org/10.1016/j.freeradbiomed.2022.01.010
Schmidt B, Ferreira C, Alves Passos CL, Silva JL, Fialho E (2020) Resveratrol, curcumin and piperine alter human glyoxalase 1 in MCF-7 breast cancer cells. Int J Mol Sci 21(15):5244. https://doi.org/10.3390/ijms21155244
Zhang W, Jiang H, Chen Y, Ren F (2019) Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J Cell Biochem 120:16283–16292. https://doi.org/10.1002/jcb.28910
Ferraz da Costa DC, Campos NPC, Santos RA, Guedes-da-Silva FH, Martins-Dinis M, Zanphorlin L et al (2018) Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget 9(49):29112–29122. https://doi.org/10.18632/oncotarget.25631
Deus CM, Serafim TL, Magalhães-Novais S, Vilaça A, Moreira AC, Sardão VA et al (2017) Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch Toxicol 91:1261–1278. https://doi.org/10.1007/s00204-016-1784-x
Gao Y, Tollefsbol TO (2018) Combinational proanthocyanidins and resveratrol synergistically inhibit human breast cancer cells and impact epigenetic-mediating machinery. Int J Mol Sci 19(8):2204. https://doi.org/10.3390/ijms19082204
Hu C, Liu Y, Teng M, Jiao K, Zhen J, Wu M et al (2019) Resveratrol inhibits the proliferation of estrogen receptor-positive breast cancer cells by suppressing EZH2 through the modulation of ERK1/2 signaling. Cell Biol Toxicol 35:445–456. https://doi.org/10.1007/s10565-019-09471-x
Dong J, Yang W, Han J, Cheng R, Li L (2020) Effects of Notch signaling components from breast cancer cells treated in culture with resveratrol. Res Vet Sci 132:369–378. https://doi.org/10.1016/j.rvsc.2020.07.017
Pan J, Shen J, Si W, Du C, Chen D, Xu L et al (2017) Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 8:65743–65758. https://doi.org/10.18632/oncotarget.19445
Kim DH, Sung B, Kim JA, Kang YJ, Hwang SY, Hwang NL et al (2017) HS-1793, a resveratrol analogue, downregulates the expression of hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human breast cancer cells in a nude mouse xenograft model. Int J Oncol 51:715–723. https://doi.org/10.3892/ijo.2017.4058
Mohapatra P, Satapathy SR, Das D, Siddharth S, Choudhuri T, Kundu CN (2014) Resveratrol mediated cell death in cigarette smoke transformed breast epithelial cells is through induction of p21Waf1/Cip1 and inhibition of long patch base excision repair pathway. Toxicol Appl Pharmacol 275(3):221–231. https://doi.org/10.1016/j.taap.2014.01.011
Izquierdo-Torres E, Rodríguez G, Meneses-Morales I, Zarain-Herzberg A (2017) ATP2A3 gene as an important player for resveratrol anticancer activity in breast cancer cells. Mol Carcinog 56:1703–1711. https://doi.org/10.1002/mc.22625
Chen Y, Cai L, Guo X, Li Z, Liao X, Zhang X et al (2021) HMGB1-activated fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma 68:71–78
Kim NH, Sung NJ, Youn HS, Park SA (2020) Gremlin-1 activates Akt/STAT3 signaling, which increases the glycolysis rate in breast cancer cells. Biochem Biophys Res Commun 533:1378–1384. https://doi.org/10.1016/j.bbrc.2020.10.025
Dou D, Ren X, Han M, Xu X, Ge X, Gu Y et al (2021) Circ_0008039 supports breast cancer cell proliferation, migration, invasion, and glycolysis by regulating the miR-140-3p/SKA2 axis. Mol Oncol 15:697–709. https://doi.org/10.1002/1878-0261.12862
Li Y, Li H, Wang W, Yu X, Xu Q (2020) LINC00346 regulates glycolysis by modulation of glucose transporter 1 in breast cancer cells. Mol Cell Probes 54:101667. https://doi.org/10.1016/j.mcp.2020.101667
Jiao L, Wang S, Zheng Y, Wang N, Yang B, Wang D et al (2019) Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-κB/c-Myc pathway. Biochem Pharmacol 161:149–162. https://doi.org/10.1016/j.bcp.2019.01.016
Wu Q, Zhao B, Sui G, Shi J (2021) Phytochemicals block glucose utilization and lipid synthesis to counteract metabolic reprogramming in cancer cells. Appl Sci 11(3):1259. https://doi.org/10.3390/app11031259
Gomez LS, Zancan P, Marcondes MC, Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M et al (2013) Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 95:1336–1343. https://doi.org/10.1016/j.biochi.2013.02.013
Erin N, Grahovac J, Brozovic A, Efferth T (2020) Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updates 53:100715. https://doi.org/10.1016/j.drup.2020.100715
Arneth B (2019) Tumor microenvironment. Medicina 56(1):15. https://doi.org/10.3390/medicina56010015
Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M et al (2020) Role of microRNA/epithelial-to-mesenchymal transition axis in the metastasis of bladder cancer. Biomolecules 10(8):1159. https://doi.org/10.3390/biom10081159
Babaei G, Aziz SG-G, Jaghi NZZJB (2021) EMT, cancer stem cells and autophagy; the three main axes of metastasis. Pharmacotherapy 133:110909. https://doi.org/10.1016/j.biopha.2020.110909
Wu Y, Sarkissyan M, Vadgama JV (2016) Epithelial-mesenchymal transition and breast cancer. J Clin Med 5(2):13. https://doi.org/10.3390/jcm5020013
Marconi GD, Fonticoli L, Rajan TS, Pierdomenico SD, Trubiani O, Pizzicannella J et al (2021) Epithelial-mesenchymal transition (EMT): the type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 10(7):1587. https://doi.org/10.3390/cells10071587
Ashrafizadeh M, Ang HL, Moghadam ER, Mohammadi S, Zarrin V, Hushmandi K et al (2020) MicroRNAs and their influence on the ZEB family: mechanistic aspects and therapeutic applications in cancer therapy. Biomolecules 10(7):1040. https://doi.org/10.3390/biom10071040
Mirzaei S, Abadi AJ, Gholami MH, Hashemi F, Zabolian A, Hushmandi K et al (2021) The involvement of epithelial-to-mesenchymal transition in doxorubicin resistance: possible molecular targets. Eur J Pharmacol 908:174344. https://doi.org/10.1016/j.ejphar.2021.174344
Sun X, Chang X, Wang Y, Xu B, Cao X (2019) Oroxylin a suppresses the cell proliferation, migration, and EMT via NF-κB signaling pathway in human breast cancer cells. Biomed Res Int 2019:9241769. https://doi.org/10.1155/2019/9241769
Sun Y, Zhou QM, Lu YY, Zhang H, Chen QL, Zhao M et al (2019) Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules 24(6):1131. https://doi.org/10.3390/molecules24061131
Yar Saglam AS, Kayhan H, Alp E, Onen HI (2021) Resveratrol enhances the sensitivity of FL118 in triple-negative breast cancer cell lines via suppressing epithelial to mesenchymal transition. Mol Biol Rep 48:475–489. https://doi.org/10.1007/s11033-020-06078-y
Ashrafizadeh M, Mirzaei S, Hashemi F, Zarrabi A, Zabolian A, Saleki H et al (2021) New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother 141:111824. https://doi.org/10.1016/j.biopha.2021.111824
Ashrafizadeh M, Zarrabi A, Hushmandi K, Kalantari M, Mohammadinejad R, Javaheri T et al (2020) Association of the epithelial-mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci 21(11):4002. https://doi.org/10.3390/ijms21114002
Yang MD, Sun Y, Zhou WJ, Xie XZ, Zhou QM, Lu YY et al (2021) Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer MDA-MB-231 cell models in vivo and in vitro. Molecules 26(8):2204. https://doi.org/10.3390/molecules26082204
Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD et al (2013) 3,5,4’-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol 272:746–756. https://doi.org/10.1016/j.taap.2013.07.019
Jung YY, Lee JH, Nam D, Narula AS, Namjoshi OA, Blough BE et al (2018) Anti-myeloma effects of icariin are mediated through the attenuation of JAK/STAT3-dependent signaling cascade. Front Pharmacol 9:531. https://doi.org/10.3389/fphar.2018.00531
Ko JH, Um JY, Lee SG, Yang WM, Sethi G, Ahn KS (2019) Conditioned media from adipocytes promote proliferation, migration, and invasion in melanoma and colorectal cancer cells. J Cell Physiol 234:18249–18261. https://doi.org/10.1002/jcp.28456
Liu D, Guo H, Li Y, Xu X, Yang K, Bai YJP (2012) Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta-analysis. PLoS ONE 7:e31251. https://doi.org/10.1371/journal.pone.0031251
Monisha J, Roy NK, Padmavathi G, Banik K, Bordoloi D, Khwairakpam AD et al (2018) NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation. Migr Chemoresist Cancers (Basel) 10(7):228. https://doi.org/10.3390/cancers10070228
Napoli S, Scuderi C, Gattuso G, Di Bella V, Candido S, Basile MS et al (2020) Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 9(5):1151. https://doi.org/10.3390/cells9051151
Balkhi S, Mashayekhi F, Salehzadeh A, Saedi HS (2020) Matrix metalloproteinase (MMP)-1 and MMP-3 gene variations affect MMP-1 and -3 serum concentration and associates with breast cancer. Mol Biol Rep 47:9637–9644. https://doi.org/10.1007/s11033-020-05962-x
Piskór BM, Przylipiak A, Dąbrowska E, Sidorkiewicz I, Niczyporuk M, Szmitkowski M et al (2020) Plasma level of MMP-10 may be a prognostic marker in early stages of breast cancer. J Clin Med 9(12):4122. https://doi.org/10.3390/jcm9124122
Shunmuga Priya V, Pradiba D, Aarthy M, Singh SK, Achary A, Vasanthi M (2021) In-silico strategies for identification of potent inhibitor for MMP-1 to prevent metastasis of breast cancer. J Biomol Struct Dyn 39:7274–7293. https://doi.org/10.1080/07391102.2020.1810776
Ozkan E, Bakar-Ates F (2020) Potentiation of the effect of lonidamine by quercetin in MCF-7 human breast cancer cells through downregulation of MMP-2/9 mRNA expression. An Acad Bras Cienc 92:e20200548. https://doi.org/10.1590/0001-3765202020200548
Tang FY, Su YC, Chen NC, Hsieh HS, Chen KS (2008) Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol Nutr Food Res 52:683–691. https://doi.org/10.1002/mnfr.200700325
Park SY, Chae SY, Park JO, Lee KJ, Park G (2016) Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol Rep 35:3248–3256. https://doi.org/10.3892/or.2016.4716
Lee HS, Ha AW, Kim WK (2012) Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr Res Pract 6:294–300. https://doi.org/10.4162/nrp.2012.6.4.294
Tang FY, Chiang EP, Sun YC (2008) Resveratrol inhibits heregulin-beta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J Nutr Biochem 19:287–294. https://doi.org/10.1016/j.jnutbio.2007.03.003
Ahn KS, Sethi G, Aggarwal BB (2008) Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem Pharmacol 75:907–913. https://doi.org/10.1016/j.bcp.2007.10.010
Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB (2006) Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem 281:19798–19808. https://doi.org/10.1074/jbc.M601162200
Morgan D, Garg M, Tergaonkar V, Tan SY, Sethi G (2020) Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim Biophys Acta Rev Cancer 1874:188449. https://doi.org/10.1016/j.bbcan.2020.188449
Puar YR, Shanmugam MK, Fan L, Arfuso F, Sethi G, Tergaonkar V (2018) Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 6(3):82. https://doi.org/10.3390/biomedicines6030082
Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A et al (2005) Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer 115:74–84. https://doi.org/10.1002/ijc.20856
Ashrafizadeh M, Gholami MH, Mirzaei S, Zabolian A, Haddadi A, Farahani MV et al (2021) Dual relationship between long non-coding RNAs and STAT3 signaling in different cancers: new insight to proliferation and metastasis. Life Sci 270:119006. https://doi.org/10.1016/j.lfs.2020.119006
Ashrafizadeh M, Zarrabi A, Orouei S, Zarrin V, Rahmani Moghadam E, Zabolian A et al (2020) STAT3 pathway in gastric cancer: signaling, therapeutic targeting and future prospects. Biology 9(6):126. https://doi.org/10.3390/biology9060126
Lee JH, Kim C, Baek SH, Ko JH, Lee SG, Yang WM et al (2017) Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 8(11):17700–17711. https://doi.org/10.18632/oncotarget.10775
Mirzaei S, Gholami MH, Mahabady MK, Nabavi N, Zabolian A, Banihashemi SM et al (2021) Pre-clinical investigation of STAT3 pathway in bladder cancer: paving the way for clinical translation. Biomed Pharmacother 133:111077. https://doi.org/10.1016/j.biopha.2020.111077
Mohan CD, Rangappa S, Preetham HD, Chandra Nayaka S, Gupta VK, Basappa S et al (2020) Targeting STAT3 signaling pathway in cancer by agents derived from mother nature. Semin Cancer Biol 80:157–182. https://doi.org/10.1016/j.semcancer.2020.03.016
Zhang J, Ahn KS, Kim C, Shanmugam MK, Siveen KS, Arfuso F et al (2016) Nimbolide-induced oxidative stress abrogates STAT3 signaling cascade and inhibits tumor growth in transgenic adenocarcinoma of mouse prostate model. Antioxid Redox Signal 24:575–589. https://doi.org/10.1089/ars.2015.6418
Kim JE, Kim HS, Shin YJ, Lee CS, Won C, Lee SA et al (2008) LYR71, a derivative of trimeric resveratrol, inhibits tumorigenesis by blocking STAT3-mediated matrix metalloproteinase 9 expression. Exp Mol Med 40:514–522. https://doi.org/10.3858/emm.2008.40.5.514
Lee MF, Pan MH, Chiou YS, Cheng AC, Huang H (2011) Resveratrol modulates MED28 (Magicin/EG-1) expression and inhibits epidermal growth factor (EGF)-induced migration in MDA-MB-231 human breast cancer cells. J Agric Food Chem 59:11853–11861. https://doi.org/10.1021/jf202426k
Maccario C, Savio M, Ferraro D, Bianchi L, Pizzala R, Pretali L et al (2012) The resveratrol analog 4,4’-dihydroxy-trans-stilbene suppresses transformation in normal mouse fibroblasts and inhibits proliferation and invasion of human breast cancer cells. Carcinogenesis 33:2172–2180. https://doi.org/10.1093/carcin/bgs244
Özdemi RF, Sever A, Keçeci Y, Incesu Z (2021) Resveratrol increases the sensitivity of breast cancer MDA-MB-231 cell line to cisplatin by regulating intrinsic apoptosis. Iran J Basic Med Sci 24:66–72. https://doi.org/10.22038/ijbms.2020.50485.11501
Azios NG, Dharmawardhane SF (2005) Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia 7(2):128–140. https://doi.org/10.1593/neo.04346
Singh B, Shoulson R, Chatterjee A, Ronghe A, Bhat NK, Dim DC et al (2014) Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 35(8):1872–1880. https://doi.org/10.1093/carcin/bgu120
Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R et al (2013) Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol 191:4141–4151. https://doi.org/10.4049/jimmunol.1300606
Han X, Zhao N, Zhu W, Wang J, Liu B, Teng Y (2021) Resveratrol attenuates TNBC lung metastasis by down-regulating PD-1 expression on pulmonary t cells and converting macrophages to M1 phenotype in a murine tumor model. Cell Immunol 368:104423. https://doi.org/10.1016/j.cellimm.2021.104423
Shibata MA, Akao Y, Shibata E, Nozawa Y, Ito T, Mishima S et al (2007) Vaticanol C, a novel resveratrol tetramer, reduces lymph node and lung metastases of mouse mammary carcinoma carrying p53 mutation. Cancer Chemother Pharmacol 60:681–691. https://doi.org/10.1007/s00280-007-0414-y
Kim YN, Choe SR, Cho KH, Cho DY, Kang J, Park CG et al (2017) Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Exp Mol Med 49:e296. https://doi.org/10.1038/emm.2016.151
Chen KY, Chen CC, Chang YC, Chang MC (2019) Resveratrol induced premature senescence and inhibited epithelial-mesenchymal transition of cancer cells via induction of tumor suppressor Rad9. PLoS ONE 14:e0219317. https://doi.org/10.1371/journal.pone.0219317
Deldar Abad Paskeh M, Asadi S, Zabolian A, Saleki H, Khoshbakht MA, Sabet S et al (2021) Targeting cancer stem cells by dietary agents: an important therapeutic strategy against human malignancies. Int J Mol Sci 22(21):11669. https://doi.org/10.3390/ijms222111669
Kirtonia A, Sethi G, Garg M (2020) The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci 77:4459–4483. https://doi.org/10.1007/s00018-020-03536-5
Ma Z, Wang YY, Xin HW, Wang L, Arfuso F, Dharmarajan A et al (2019) The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int J Biochem Cell Biol 108:17–20. https://doi.org/10.1016/j.biocel.2019.01.003
Warrier S, Patil M, Bhansali S, Varier L, Sethi G (2021) Designing precision medicine panels for drug refractory cancers targeting cancer stemness traits. Biochim Biophys Acta Rev Cancer 1875:188475. https://doi.org/10.1016/j.bbcan.2020.188475
Safaeinejad Z, Kazeminasab F, Kiani-Esfahani A, Ghaedi K, Nasr-Esfahani MH (2018) Multi-effects of resveratrol on stem cell characteristics: effective dose, time, cell culture conditions and cell type-specific responses of stem cells to resveratrol. Eur J Med Chem 155:651–657. https://doi.org/10.1016/j.ejmech.2018.06.037
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y et al (2014) Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS ONE 9:e102535. https://doi.org/10.1371/journal.pone.0102535
Suh J, Kim DH, Surh YJ (2018) Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys 643:62–71. https://doi.org/10.1016/j.abb.2018.02.011
Dewangan J, Tandon D, Srivastava S, Verma AK, Yapuri A, Rath SK (2017) Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis 22:1246–1259. https://doi.org/10.1007/s10495-017-1394-y
Lainetti PF, Leis-Filho AF, Laufer-Amorim R, Battazza A, Fonseca-Alves CEJP (2022) Mechanisms of resistance to chemotherapy in breast cancer and possible targets in drug delivery systems. Pharmaceutics 12(12):1193. https://doi.org/10.3390/pharmaceutics12121193
Lee JH, Chiang SY, Nam D, Chung WS, Lee J, Na YS et al (2014) Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett 345(1):140–148. https://doi.org/10.1016/j.canlet.2013.12.008
Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP et al (2012) First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin Cancer Res 18(8):2220–2229. https://doi.org/10.1158/1078-0432.CCR-11-2470
Pandya G, Kirtonia A, Sethi G, Pandey AK (1874) Garg M (2020) The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim Biophys Acta Rev Cancer 2:188423. https://doi.org/10.1016/j.bbcan.2020.188423
Garg M, Shanmugam MK, Bhardwaj V, Goel A, Gupta R, Sharma A et al (2020) The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy. Med Res Rev 41(3):1291–1336. https://doi.org/10.1002/med.21761
Gupta B, Sadaria D, Warrier VU, Kirtonia A, Kant R, Awasthi A et al (2020) Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin Cancer Biol 80:87–106. https://doi.org/10.1016/j.semcancer.2020.02.005
Ma Z, Wang LZ, Cheng JT, Lam WST, Ma X, Xiang X et al (2021) Targeting hypoxia-inducible factor-1-mediated metastasis for cancer therapy. Antioxid Redox Signal 34:1484–1497. https://doi.org/10.1089/ars.2019.7935
Raghunath A, Sundarraj K, Arfuso F, Sethi G, Perumal E (2018) Dysregulation of Nrf2 in hepatocellular carcinoma: role in cancer progression and chemoresistance. Cancers (Basel) 10(12):481. https://doi.org/10.3390/cancers10120481
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B (2017) The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull 7(3):339–348. https://doi.org/10.15171/apb.2017.041
Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E (2018) The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis 35:309–318. https://doi.org/10.1007/s10585-018-9903-0
Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC (2019) Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother 114:108800. https://doi.org/10.1016/j.biopha.2019.108800
Bai X, Ni J, Beretov J, Graham P, Li Y (2021) Triple-negative breast cancer therapeutic resistance: where is the Achilles’ heel? Cancer Lett 497:100–111. https://doi.org/10.1016/j.canlet.2020.10.016
Kamble D, Mahajan M, Dhat R, Sitasawad S (2021) Keap1-Nrf2 pathway regulates ALDH and contributes to radioresistance in breast cancer stem cells. Cells 10(1):83. https://doi.org/10.3390/cells10010083
Qiu L, Ma Y, Chen X, Zhou L, Zhang H, Zhong G et al (2021) Heparin-binding growth factor (HDGF) drives radioresistance in breast cancer by activating the STAT3 signaling pathway. J Transl Med 19:344. https://doi.org/10.1186/s12967-021-03021-y
Li J, Lei C, Chen B, Zhu Q (2021) LncRNA FGD5-AS1 facilitates the radioresistance of breast cancer cells by enhancing MACC1 expression through competitively sponging miR-497-5p. Front Oncol 11:671853. https://doi.org/10.3389/fonc.2021.671853
Ashrafizadeh M, Mirzaei S, Gholami MH, Hashemi F, Zabolian A, Raei M et al (2021) Hyaluronic acid-based nanoplatforms for doxorubicin: a review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr Polym 272:118491. https://doi.org/10.1016/j.carbpol.2021.118491
Ashrafizaveh S, Ashrafizadeh M, Zarrabi A, Husmandi K, Zabolian A, Shahinozzaman M et al (2021) Long non-coding RNA in the doxorubicin resistance of cancer cells. Cancer Lett 508:104–114. https://doi.org/10.1016/j.canlet.2021.03.018
Mirzaei S, Gholami MH, Hashemi F, Zabolian A, Farahani MV, Hushmandi K et al (2021) Advances in understanding the role of P-gp in doxorubicin resistance: molecular pathways, therapeutic strategies, and prospects. Drug Discov Today 27(2):436–455. https://doi.org/10.1016/j.drudis.2021.09.020
Mitani T, Ito Y, Harada N, Nakano Y, Inui H, Ashida H et al (2014) Resveratrol reduces the hypoxia-induced resistance to doxorubicin in breast cancer cells. J Nutr Sci Vitaminol (Tokyo) 60:122–128. https://doi.org/10.3177/jnsv.60.122
Huang F, Wu XN, Chen J, Wang WX, Lu ZF (2014) Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med 7:1611–1616. https://doi.org/10.3892/etm.2014.1662
Kim TH, Shin YJ, Won AJ, Lee BM, Choi WS, Jung JH et al (2014) Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim Biophys Acta 1840:615–625. https://doi.org/10.1016/j.bbagen.2013.10.023
Jin X, Wei Y, Liu Y, Lu X, Ding F, Wang J et al (2019) Resveratrol promotes sensitization to Doxorubicin by inhibiting epithelial-mesenchymal transition and modulating SIRT1/β-catenin signaling pathway in breast cancer. Cancer Med 8(3):1246–1257. https://doi.org/10.1002/cam4.1993
Díaz-Chávez J, Fonseca-Sánchez MA, Arechaga-Ocampo E, Flores-Pérez A, Palacios-Rodríguez Y, Domínguez-Gómez G et al (2013) Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 8:e64378. https://doi.org/10.1371/journal.pone.0064378
Abadi AJ, Zarrabi A, Gholami MH, Mirzaei S, Hashemi F, Zabolian A et al (2021) Small in size, but large in action: microRNAs as potential modulators of PTEN in breast and lung cancers. Biomolecules 11(2):304. https://doi.org/10.3390/biom11020304
Ashrafizadeh M, Najafi M, Ang HL, Moghadam ER, Mahabady MK, Zabolian A et al (2020) PTEN, a barrier for proliferation and metastasis of gastric cancer cells: from molecular pathways to targeting and regulation. Biomedicines 8(8):264. https://doi.org/10.3390/biomedicines8080264
Ashrafizadeh M, Zarrabi A, Samarghandian S, Najafi MJEJoP (2020) PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol 881:173226. https://doi.org/10.1016/j.ejphar.2020.173226
Chen JM, Bai JY, Yang KX (2018) Effect of resveratrol on doxorubicin resistance in breast neoplasm cells by modulating PI3K/Akt signaling pathway. IUBMB Life 70(6):491–500. https://doi.org/10.1002/iub.1749
Mirzaei S, Hushmandi K, Zabolian A, Saleki H, Torabi SMR, Ranjbar A et al (2021) Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: a focus on molecular pathways and possible therapeutic strategies. Molecules 26(8):2382. https://doi.org/10.3390/molecules26082382
Leon-Galicia I, Diaz-Chavez J, Albino-Sanchez ME, Garcia-Villa E, Bermudez-Cruz R, Garcia-Mena J et al (2018) Resveratrol decreases Rad51 expression and sensitizes cisplatin-resistant MCF-7 breast cancer cells. Oncol Rep 39:3025–3033. https://doi.org/10.3892/or.2018.6336
Shi XP, Miao S, Wu Y, Zhang W, Zhang XF, Ma HZ et al (2013) Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. Int J Mol Sci 14(8):15655–15668. https://doi.org/10.3390/ijms140815655
Sprouse AA, Herbert BS (2014) Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer Res 34:5363–5374
Vinod BS, Nair HH, Vijayakurup V, Shabna A, Shah S, Krishna A et al (2015) Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis. Cell Death Discov 1:15061. https://doi.org/10.1038/cddiscovery.2015.61
Choi YJ, Heo K, Park HS, Yang KM, Jeong MH (2016) The resveratrol analog HS-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions. Int J Oncol 49:1479–1488. https://doi.org/10.3892/ijo.2016.3647
Aghamiri S, Jafarpour A, Zandsalimi F, Aghemiri M, Shoja M (2019) Effect of resveratrol on the radiosensitivity of 5-FU in human breast cancer MCF-7 cells. J Cell Biochem 120:15671–15677. https://doi.org/10.1002/jcb.28836
da Costa Araldi IC, Bordin FPR, Cadoná FC, Barbisan F, Azzolin VF, Teixeira CF et al (2018) The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem Biol Interact 282:85–92. https://doi.org/10.1016/j.cbi.2018.01.013
Leon-Galicia I, Diaz-Chavez J, Albino-Sanchez ME, Garcia-Villa E, Bermudez-Cruz R, Garcia-Mena J, Herrera LA, García-Carrancá A, Gariglio P (2018) Resveratrol decreases Rad51 expression and sensitizes cisplatin-resistant MCF-7 breast cancer cells. Oncol Rep 39(6):3025–3033. https://doi.org/10.3892/or.2018.6336
Abdel-Latif GA, Al-Abd AM, Tadros MG, Al-Abbasi FA, Khalifa AE, Abdel-Naim AB (2015) The chemomodulatory effects of resveratrol and didox on herceptin cytotoxicity in breast cancer cell lines. Sci Rep 5(1):1–13. https://doi.org/10.1038/srep12054
Arora D, Jaglan S (2017) Nanocarriers for resveratrol delivery. Nanoscience in food and agriculture, vol 5. Springer, Berlin, pp 123–138
Summerlin N, Soo E, Thakur S, Qu Z, Jambhrunkar S, Popat A (2015) Resveratrol nanoformulations: challenges and opportunities. Int J Pharm 479(2):282–290. https://doi.org/10.1016/j.ijpharm.2015.01.003
Elzoghby AO, El-Lakany SA, Helmy MW, Abu-Serie MM, Elgindy NA (2017) Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine (Lond) 12:2785–2805. https://doi.org/10.2217/nnm-2017-0247
Luo X, Wang D, Wang M, Deng S, Huang Y, Xia Z (2021) Development of phospholipid complex loaded self-microemulsifying drug delivery system to improve the oral bioavailability of resveratrol. Nanomedicine (Lond) 16:721–739. https://doi.org/10.2217/nnm-2020-0422
Shi Q, Wang X, Tang X, Zhen N, Wang Y, Luo Z et al (2021) In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci Nutr 9:3530–3537. https://doi.org/10.1002/fsn3.2302
Thipe VC, Panjtan Amiri K, Bloebaum P, Raphael Karikachery A, Khoobchandani M, Katti KK et al (2019) Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomed 14:4413–4428. https://doi.org/10.2147/IJN.S204443
Zhang X, Han L, Sun Q, Xia W, Zhou Q, Zhang Z et al (2020) Controlled release of resveratrol and xanthohumol via coaxial electrospinning fibers. J Biomater Sci Polym Ed 31:456–471. https://doi.org/10.1080/09205063.2019.1700600
Zhao YN, Cao YN, Sun J, Liang Z, Wu Q, Cui SH et al (2020) Anti-breast cancer activity of resveratrol encapsulated in liposomes. J Mater Chem B 8:27–37. https://doi.org/10.1039/C9TB02051A
Gregoriou Y, Gregoriou G, Yilmaz V, Kapnisis K, Prokopi M, Anayiotos A et al (2021) Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 5(1):113–124. https://doi.org/10.7150/ntno.51955
El-Far SW, Helmy MW, Khattab SN, Bekhit AA, Hussein AA, Elzoghby AO (2018) Phytosomal bilayer-enveloped casein micelles for codelivery of monascus yellow pigments and resveratrol to breast cancer. Nanomedicine (Lond) 13:481–499. https://doi.org/10.2217/nnm-2017-0301
Guo X, Zhao Z, Chen D, Qiao M, Wan F, Cun D et al (2019) Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J Pharm Sci 14(1):78–85. https://doi.org/10.1016/j.ajps.2018.03.002
Gadag S, Narayan R, Nayak AS, Catalina Ardila D, Sant S, Nayak Y et al (2021) Development and preclinical evaluation of microneedle-assisted resveratrol loaded nanostructured lipid carriers for localized delivery to breast cancer therapy. Int J Pharm 606:120877. https://doi.org/10.1016/j.ijpharm.2021.120877
Poonia N, Kaur Narang J, Lather V, Beg S, Sharma T, Singh B et al (2019) Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B Biointerfaces 181:756–766. https://doi.org/10.1016/j.colsurfb.2019.06.004
Catania A, Barrajón-Catalán E, Nicolosi S, Cicirata F, Micol V (2013) Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res Treat 141(1):55–65. https://doi.org/10.1007/s10549-013-2667-y
Xu X, Liu A, Bai Y, Li Y, Zhang C, Cui S et al (2019) Co-delivery of resveratrol and p53 gene via peptide cationic liposomal nanocarrier for the synergistic treatment of cervical cancer and breast cancer cells. J Drug Deliv Sci Technol 51:746–753. https://doi.org/10.1016/j.jddst.2018.05.008
Hai L, Zhang A, Wu X, Cheng H, He D, Wang T, He X, Wang K (2019) Liposome-stabilized black phosphorus for photothermal drug delivery and oxygen self-enriched photodynamic therapy. ACS Appl Nano Mater 3(1):563–575. https://doi.org/10.1021/acsanm.9b02119
Shrivastava N, Parikh A, Dewangan RP, Biswas L, Verma AK, Mittal S, Ali J, Garg S, Baboota S (2022) Solid self-nano emulsifying nanoplatform loaded with tamoxifen and resveratrol for treatment of breast cancer. Pharmaceutics 14(7):1486. https://doi.org/10.3390/pharmaceutics14071486
Ashrafizade M, Delfi M, Hashemi F, Zabolian A, Saleki H, Bagherian M et al (2021) Biomedical application of chitosan-based nanoscale delivery systems: potential usefulness in siRNA delivery for cancer therapy. Carbohydr Polym 260:117809. https://doi.org/10.1016/j.carbpol.2021.117809
Makvandi P, Ghomi M, Ashrafizadeh M, Tafazoli A, Agarwal T, Delfi M et al (2020) A review on advances in graphene-derivative/polysaccharide bionanocomposites: therapeutics, pharmacogenomics and toxicity. Carbohydr Polym 250:116952. https://doi.org/10.1016/j.carbpol.2020.116952
Al-Jubori AA, Sulaiman GM, Tawfeeq AT, Mohammed HA, Khan RA, Mohammed SAA (2021) Layer-by-layer nanoparticles of tamoxifen and resveratrol for dual drug delivery system and potential triple-negative breast cancer treatment. Pharmaceutics 13(7):1098. https://doi.org/10.3390/pharmaceutics13071098
Zhao Y, Cai C, Liu M, Zhao Y, Wu Y, Fan Z et al (2020) Drug-binding albumins forming stabilized nanoparticles for co-delivery of paclitaxel and resveratrol: in vitro/in vivo evaluation and binding properties investigation. Int J Biol Macromol 153:873–882. https://doi.org/10.1016/j.ijbiomac.2020.03.060
Kumar S, Lather V, Pandita D (2016) A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery. Int J Biol Macromol 84:380–384. https://doi.org/10.1016/j.ijbiomac.2015.12.036
Fan C, Kong F, Shetti D, Zhang B, Yang Y, Wei K (2019) Resveratrol loaded oxidized mesoporous carbon nanoparticles: a promising tool to treat triple negative breast cancer. Biochem Biophys Res Commun 519:378–384. https://doi.org/10.1016/j.bbrc.2019.09.016
Lin J-T, Du J-K, Yang Y-Q, Li L, Zhang D-W, Liang C-L et al (2017) pH and redox dual stimulate-responsive nanocarriers based on hyaluronic acid coated mesoporous silica for targeted drug delivery. Mater Sci Eng C 81:478–484. https://doi.org/10.1016/j.msec.2017.08.036
Tayo LLJB (2017) Stimuli-responsive nanocarriers for intracellular delivery. Biophys Rev 9:931–940. https://doi.org/10.1007/s12551-017-0341-z
Wu M, Cao Z, Zhao Y, Zeng R, Tu M, Zhao JJMS et al (2016) Novel self-assembled pH-responsive biomimetic nanocarriers for drug delivery. Mater Sci Eng C 64:346–353. https://doi.org/10.1016/j.msec.2016.03.099
Jing T, Li T, Ruan Z, Yan LJJoMS (2018) pHe-and glutathione-stepwise-responsive polypeptide nanogel for smart and efficient drug delivery. J Mater Sci 53(21):14933–14943. https://doi.org/10.1007/s10853-018-2689-2
Arunachalam B, Phan UT, Geuze HJ, Cresswell P (2000) Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci USA 97:745–750. https://doi.org/10.1073/pnas.97.2.745
Daga M, Ullio C, Argenziano M, Dianzani C, Cavalli R, Trotta F et al (2016) GSH-targeted nanosponges increase doxorubicin-induced toxicity “in vitro” and “in vivo” in cancer cells with high antioxidant defenses. Free Radic Biol Med 97:24–37. https://doi.org/10.1016/j.freeradbiomed.2016.05.009
Palminteri M, Dhakar NK, Ferraresi A, Caldera F, Vidoni C, Trotta F et al (2021) Cyclodextrin nanosponge for the GSH-mediated delivery of Resveratrol in human cancer cells. Nanotheranostics 5:197–212. https://doi.org/10.7150/ntno.53888
Melo BL, Lima-Sousa R, Alves CG, Moreira AF, Correia IJ, de Melo-Diogo D (2022) Chitosan-based injectable in situ forming hydrogels containing dopamine-reduced graphene oxide and resveratrol for breast cancer chemo-photothermal therapy. Biochem Eng J 185:108529. https://doi.org/10.1016/j.bej.2022.108529
Fan C, Kong F, Shetti D, Zhang B, Yang Y, Wei K (2019) Resveratrol loaded oxidized mesoporous carbon nanoparticles: A promising tool to treat triple negative breast cancer. Biochem Biophys Res Commun 519(2):378–384. https://doi.org/10.1016/j.bbrc.2019.09.016
Hai L, He D, He X, Wang K, Yang X, Liu J et al (2017) Facile fabrication of a resveratrol loaded phospholipid@reduced graphene oxide nanoassembly for targeted and near-infrared laser-triggered chemo/photothermal synergistic therapy of cancer in vivo. J Mater Chem B 5:5783–5792. https://doi.org/10.1039/C7TB01600J
Venditti I, Iucci G, Fratoddi I, Cipolletti M, Montalesi E, Marino M et al (2020) Direct conjugation of resveratrol on hydrophilic gold nanoparticles: structural and cytotoxic studies for biomedical applications. Nanomaterials (Basel) 10(10):1898. https://doi.org/10.3390/nano10101898
Ren B, Kwah MXY, Liu C, Ma Z, Shanmugam MK, Ding L, Xiang X, Ho PCL, Wang L, Ong PS, Goh BC (2021) Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett 515:63–72. https://doi.org/10.1016/j.canlet.2021.05.001
Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B, Sauter ER (2012) Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer 64(3):393–400. https://doi.org/10.1080/01635581.2012.654926
Amiot MJ, Romier B, Dao TMA, Fanciullino R, Ciccolini J, Burcelin R, Pechere L, Emond C, Savouret JF, Seree E (2013) Optimization of trans-resveratrol bioavailability for human therapy. Biochimie 95(6):1233–1238. https://doi.org/10.1016/j.biochi.2013.01.008
Alamolhodaei NS, Tsatsakis AM, Ramezani M, Hayes AW, Karimi G (2017) Resveratrol as MDR reversion molecule in breast cancer: An overview. Food Chem Toxicol 103:223–232. https://doi.org/10.1016/j.fct.2017.03.024
Bhaskara VK, Mittal B, Mysorekar VV, Amaresh N, Simal-Gandara J (2020) Resveratrol, cancer and cancer stem cells: a review on past to future. Curr Res Food Sci 3:284–295. https://doi.org/10.1016/j.crfs.2020.10.004
Funding
None.
Author information
Authors and Affiliations
Contributions
MB: conceptualization, investigation, writing—original draft; MD: methodology, investigation, writing—original draft; AZ: conceptualization, investigation, writing—original draft; DK: methodology, investigation; SJ: investigation, writing—original draft; FHS: methodology, writing—original draft; MH: conceptualization, writing—original draft; TT: investigation, writing—original draft; MR: investigation, writing—original draft; SM: methodology, writing—original draft; AZ: investigation, writing—review and editing; AZ: writing—review and editing, supervision; DDG: writing—review and editing; AB: writing—review and editing, supervision, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behroozaghdam, M., Dehghani, M., Zabolian, A. et al. Resveratrol in breast cancer treatment: from cellular effects to molecular mechanisms of action. Cell. Mol. Life Sci. 79, 539 (2022). https://doi.org/10.1007/s00018-022-04551-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04551-4