Abstract
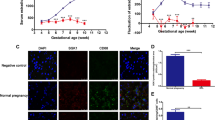
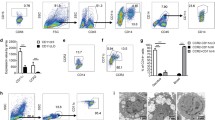
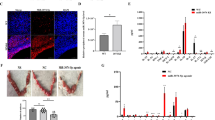
During embryo implantation, apoptosis is inevitable. These apoptotic cells (ACs) are removed by efferocytosis, in which macrophages are filled with a metabolite load nearly equal to the phagocyte itself. A timely question pertains to the relationship between efferocytosis-related metabolism and the immune behavior of decidual macrophages (dMΦs) and its effect on pregnancy outcome. Here, we report positive feedback of IL-33/ST2-AXL-efferocytosis leading to pregnancy failure through metabolic reprogramming of dMΦs. We compared the serum levels of IL-33 and sST2, along with IL-33 and ST2, efferocytosis and metabolism of dMΦs, from patients with normal pregnancies and unexplained recurrent pregnancy loss (RPL). We revealed disruption of the IL-33/ST2 axis, increased apoptotic cells and elevated efferocytosis of dMΦs from patients with RPL. The dMΦs that engulfed many apoptotic cells secreted more sST2 and less TGF-β, which polarized dMΦs toward the M1 phenotype. Moreover, the elevated sST2 biased the efferocytosis-related metabolism of RPL dMΦs toward oxidative phosphorylation and exacerbated the disruption of the IL-33/ST2 signaling pathway. Metabolic disorders also lead to dysfunction of efferocytosis, resulting in more uncleared apoptotic cells and secondary necrosis. We also screened the efferocytotic molecule AXL regulated by IL-33/ST2. This positive feedback axis of IL-33/ST2-AXL-efferocytosis led to pregnancy failure. IL-33 knockout mice demonstrated poor pregnancy outcomes, and exogenous supplementation with mouse IL-33 reduced the embryo losses. These findings highlight a new etiological mechanism whereby dMΦs leverage immunometabolism for homeostasis of the microenvironment at the maternal–fetal interface.








Similar content being viewed by others
Availability of data and material
Not applicable.
References
Doran AC, Yurdagul A, Tabas I (2020) Efferocytosis in health and disease. Nat Rev Immunol 20(4):254–267. https://doi.org/10.1038/s41577-019-0240-6
Boada-Romero E, Martinez J, Heckmann BL et al (2020) The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol 21(7):398–414. https://doi.org/10.1038/s41580-020-0232-1
Zhou Y, Yao Y, Deng Y et al (2020) Regulation of efferocytosis as a novel cancer therapy. Cell Commun Signal 18(1):71. https://doi.org/10.1186/s12964-020-00542-9
Von Rango U, Krusche CA, Kertschanska S et al (2003) Apoptosis of extravillous trophoblast cells limits the trophoblast invasion in uterine but not in tubal pregnancy during first trimester. Placenta 24(10):929–940. https://doi.org/10.1016/s0143-4004(03)00168-1
Hoijman E, Häkkinen H-M, Tolosa-Ramon Q et al (2021) Cooperative epithelial phagocytosis enables error correction in the early embryo. Nature 590(7847):618–623. https://doi.org/10.1038/s41586-021-03200-3
Sheng YR, Hu WT, Wei CY et al (2019) Insights of efferocytosis in normal and pathological pregnancy. Am J Reprod Immunol 82(2):e13088. https://doi.org/10.1111/aji.13088
Xu H, Li D, Ma J et al (2021) The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization. Cancer Biol Med 18(1):172–183. https://doi.org/10.20892/j.issn.2095-3941.2020.0211
Fock V, Mairhofer M, Otti GR et al (2013) Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol 191(7):3734–3743. https://doi.org/10.4049/jimmunol.1300490
Hu WT, Li MQ, Liu W et al (2014) IL-33 enhances proliferation and invasiveness of decidual stromal cells by up-regulation of CCL2/CCR2 via NF-κB and ERK1/2 signaling. Mol Hum Reprod 20(4):358–372. https://doi.org/10.1093/molehr/gat094
Hu WT, Huang LL, Li MQ et al (2015) Decidual stromal cell-derived IL-33 contributes to Th2 bias and inhibits decidual NK cell cytotoxicity through NF-κB signaling in human early pregnancy. J Reprod Immunol 109:52–65. https://doi.org/10.1016/j.jri.2015.01.004
Kaitu’U-lino TJ, Tuohey L, Tong S (2012) Maternal serum interleukin-33 and soluble ST2 across early pregnancy, and their association with miscarriage. J Reprod Immunol 95(1–2):46–49. https://doi.org/10.1016/j.jri.2012.06.003
Taniguchi S, Elhance A, van Duzer A et al (2020) Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science 369:6501. https://doi.org/10.1126/science.aay1813
Xu H, Sun L, He Y et al (2019) Deficiency in IL-33/ST2 Axis Reshapes Mitochondrial Metabolism in Lipopolysaccharide-Stimulated Macrophages. Front Immunol 10:127. https://doi.org/10.3389/fimmu.2019.00127
van Dijk MM, Kolte AM, Limpens J et al (2020) Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update 26(3):356–367. https://doi.org/10.1093/humupd/dmz048
Sheng YR, Hu WT, Wei CY et al (2018) IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am J Reprod Immunol 79(6):e12836. https://doi.org/10.1111/aji.12836
Zhang S, Weinberg S, Deberge M et al (2019) Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab 29(2):443–456. https://doi.org/10.1016/j.cmet.2018.12.004
Morioka S, Perry JSA, Raymond MH et al (2018) Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563(7733):714–718. https://doi.org/10.1038/s41586-018-0735-5
Li Y, Yan J, Chang HM et al (2021) Roles of TGF-β superfamily proteins in extravillous trophoblast invasion. Trends Endocrinol Metab 32(3):170–189. https://doi.org/10.1016/j.tem.2020.12.005
Qin S, Zhang Y, Zhang J et al (2020) SPRY4 regulates trophoblast proliferation and apoptosis via regulating IFN-γ-induced STAT1 expression and activation in recurrent miscarriage. Am J Reprod Immunol 83(6):e13234. https://doi.org/10.1111/aji.13234
Davra V, Kumar S, Geng K et al (2020) Axl and Mertk receptors cooperate to promote breast cancer progression by combined oncogenic signaling and evasion of host anti-tumor immunity. Can Res. https://doi.org/10.1158/0008-5472.Can-20-2066
Tanaka M, Siemann DW (2020) Gas6/Axl signaling pathway in the tumor immune microenvironment. Cancers 12:7. https://doi.org/10.3390/cancers12071850
Myers KV, Amend SR, Pienta KJ (2019) Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer 18(1):94. https://doi.org/10.1186/s12943-019-1022-2
Yang X, Liu H, Ye T et al (2020) AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization. Theranostics 10(26):12011–12025. https://doi.org/10.7150/thno.51144
Shinde R, Hezaveh K, Halaby MJ et al (2018) Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat Immunol 19(6):571–582. https://doi.org/10.1038/s41590-018-0107-1
Campesato LF, Budhu S, Tchaicha J et al (2020) Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun 11(1):4011. https://doi.org/10.1038/s41467-020-17750-z
Paparini D, Grasso E, Calo G et al (2015) Trophoblast cells primed with vasoactive intestinal peptide enhance monocyte migration and apoptotic cell clearance through αvβ3 integrin portal formation in a model of maternal-placental interaction. Mol Hum Reprod 21(12):930–941. https://doi.org/10.1093/molehr/gav059
Akhtar N, Li W, Mironov A et al (2016) Rac1 controls both the secretory function of the mammary gland and its remodeling for successive gestations. Dev Cell 38(5):522–535. https://doi.org/10.1016/j.devcel.2016.08.005
Westman J, Grinstein S, Marques PE (2019) Phagocytosis of necrotic debris at sites of injury and inflammation. Front Immunol 10:3030. https://doi.org/10.3389/fimmu.2019.03030
Gerlach BD, Marinello M, Heinz J et al (2020) Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ 27(2):525–539. https://doi.org/10.1038/s41418-019-0370-1
Abrahams VM, Kim YM, Straszewski SL et al (2004) Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol 51(4):275–282. https://doi.org/10.1111/j.1600-0897.2004.00156.x
Chamley LW, Chen Q, Ding J et al (2011) Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol 88(2):99–105. https://doi.org/10.1016/j.jri.2011.01.002
Sun F, Wang S, Du M (2021) Functional regulation of decidual macrophages during pregnancy. J Reprod Immunol 143:103264. https://doi.org/10.1016/j.jri.2020.103264
van den Bossche J, O’Neill LA, Menon D (2017) Macrophage Immunometabolism: where are we (going)? Trends Immunol 38(6):395–406. https://doi.org/10.1016/j.it.2017.03.001
Batista-Gonzalez A, Vidal R, Criollo A et al (2019) New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front Immunol 10:2993. https://doi.org/10.3389/fimmu.2019.02993
Meng Q, Guo P, Jiang Z et al (2020) Dexmedetomidine inhibits LPS-induced proinflammatory responses via suppressing HIF1α-dependent glycolysis in macrophages. Aging 12(10):9534–9548. https://doi.org/10.18632/aging.103226
Korbecki J, Bajdak-Rusinek K (2019) The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res 68(11):915–932. https://doi.org/10.1007/s00011-019-01273-5
Peng S, Sun M, Sun X et al (2018) Plasma levels of TAM receptors and ligands in severe preeclampsia. Pregn Hypertens 13:116–120. https://doi.org/10.1016/j.preghy.2018.05.012
Retallack H, di Lullo E, Arias C et al (2016) Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci USA 113(50):14408–14413. https://doi.org/10.1073/pnas.1618029113
Hastings AK, Yockey LJ, Jagger BW et al (2017) TAM receptors are not required for zika virus infection in mice. Cell Rep 19(3):558–568. https://doi.org/10.1016/j.celrep.2017.03.058
Wells MF, Salick MR, Wiskow O et al (2016) Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell 19(6):703–708. https://doi.org/10.1016/j.stem.2016.11.011
Ryan N, Anderson K, Volpedo G et al (2020) The IL-33/ST2 axis in immune responses against parasitic disease: potential therapeutic applications. Front Cell Infect Microbiol 10:153. https://doi.org/10.3389/fcimb.2020.00153
Li Z, Zhou G, Jiang L et al (2018) Effect of STOX1 on recurrent spontaneous abortion by regulating trophoblast cell proliferation and migration via the PI3K/AKT signaling pathway. J Cell Biochem 120(5):8291–8299. https://doi.org/10.1002/jcb.28112
Ding J, Yang C, Zhang Y et al (2021) M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J Cell Mol Med 25(4):2136–2147. https://doi.org/10.1111/jcmm.16191
Huang J, Xue M, Zhang J et al (2019) Protective role of GPR120 in the maintenance of pregnancy by promoting decidualization via regulation of glucose metabolism. EBioMedicine 39:540–551. https://doi.org/10.1016/j.ebiom.2018.12.019
Homsak E, Gruson D (2020) Soluble ST2: a complex and diverse role in several diseases. Clin Chim Acta 507:75–87. https://doi.org/10.1016/j.cca.2020.04.011
Granne I, Southcombe JH, Snider JV et al (2011) ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS ONE 6(9):e24463. https://doi.org/10.1371/journal.pone.0024463
Zhu C, Wei Y, Wei X (2019) AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer 18(1):153. https://doi.org/10.1186/s12943-019-1090-3
Acknowledgements
All authors thank all the participants involved in this study, especially Miss Taniya from Albania for editing the English language and spelling.
Funding
This research was funded by the National Key R&D Program of China (2021YFC2701602), the National Natural Science Foundation of China (NSFC 81871143, 82071624, 81671457, 31800768, 92057119, and 31970798), the Program for Zhuoxue of Fudan University (JIF157602) and the Support Project for Original Personalized Research of Fudan University.
Author information
Authors and Affiliations
Contributions
Y-RS performed the experiments and data analysis, generated the figures and prepared the manuscript; W‑TH designed the study, performed experiments and assisted with data interpretation; H-HS performed experiments, searched the literature and edited the manuscript; C‑YW, Y‑KL, X-QM searched the literature; M-QL designed the study, guided the experiments and edited the manuscript; X-YZ initiated and supervised the study and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
Tissue samples used in this study were collected after obtaining informed consent approved by the Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University.
Consent for publication
All authors agree to publish in the journal Cellular and Molecular Life Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sheng, Y., Hu, W., Shen, HH. et al. An imbalance of the IL-33/ST2-AXL-efferocytosis axis induces pregnancy loss through metabolic reprogramming of decidual macrophages. Cell. Mol. Life Sci. 79, 173 (2022). https://doi.org/10.1007/s00018-022-04197-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04197-2