Abstract
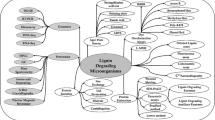
Lignocellulosic materials form the building block of 50% of plant biomass comprising non-chewable agri-components like wheat straw, rice stubbles, wood shavings and other crop residues. The degradation of lignin, cellulose and hemicellulose is complicated and presently being done by chemical process for industrial application through a very energy intensive process. Lignin degradation is primarily an oxidative process where the enzyme lignin peroxidase digests the polymer into smaller fragments. Being a recalcitrant component, higher lignin content poses a challenge of lower recovery of product for industrial use. Globally, the scientists are working on leveraging fungal biotechnology for using the lignocellulose degrading enzymes secreted by actinomycetes and basidiomycetes fungal groups. Enzymes contributing to degradation of lignin are mainly performing the function of modifying the lignin and degrading the lignin. Ligninolytic enzymes do not act as an independent reaction but are vital to complete the degradation process. Microbial enzyme technology is an emerging green tool in industrial biotechnology for commercial application. Bioprocessing of lignocellulosic biomass is challenged by limitations in enzymatic and conversion process where pretreatment and separation steps are done to remove lignin and hydrolyze carbohydrate into fermentable sugars. This review highlights recent advances in molecular biotechnology, lignin valorization, sequencing, decipher microbial membership, and characterize enzyme diversity through ‘omics’ techniques. Emerging techniques to characterize the interwoven metabolism and spatial interactions between anaerobes are also reviewed, which will prove critical to developing a predictive understanding of anaerobic communities to guide in microbiome engineering This requires more synergistic collaborations from microbial biotechnologists, bioprocess engineers, enzymologists, and other biotechnological fields.















Similar content being viewed by others
Data availability
Since it is a review article, the availability of data and material is not applicable.
Change history
28 February 2022
Affiliation has been updated as per author request
References
Soltanian S et al (2020) A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers Manag 212:112792. https://doi.org/10.1016/j.enconman.2020.112792
de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119. https://doi.org/10.1016/j.jbiotec.2016.08.011
Del Río JC, Marques G, Rencoret J, Martínez ÁT, Gutiérrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55(14):5461–5468. https://doi.org/10.1021/jf0705264
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6(2):e03170. https://doi.org/10.1016/j.heliyon.2020.e03170
Mirmohamadsadeghi S et al (2021) Pretreatment of lignocelluloses for enhanced biogas production: a review on influencing mechanisms and the importance of microbial diversity. Renew Sustain Energy Rev 135:110173. https://doi.org/10.1016/j.rser.2020.110173
Nadar SS, Rao P, Rathod VK (2018) “Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: a review.” Food Res Int 108:309–330. https://doi.org/10.1016/j.foodres.2018.03.006
Yimer D, Tilahun A (2018) Microbial biotechnology review in microbial enzyme production methods, assay techniques and protein separation and purifications. J Nutr Health Food Eng 8(1):1–7
Kumar A, Srivastva D, Chand R (2019) Rapid bioconversion of lignocellulosic biomass by fungi. Mycodegradation of Lignocelluloses. Springer, pp 137–165
Adrio JL, Demain AL (2014) Microbial enzymes: tools for biotechnological processes. Biomolecules. https://doi.org/10.3390/biom4010117
Kyrpides NC et al (2014) Genomic encyclopedia of bacteria and archaea: sequencing a myriad of type strains. PLoS Biol 12(8):e1001920
López-Mondéjar R, Zühlke D, Becher D, Riedel K, Baldrian P (2016) Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci Rep 6(1):1–12
Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. Int Sch Res Notices. https://doi.org/10.5402/2013/985685
Chandra R (2015) Advances in biodegradation and bioremediation of industrial waste. CRC Press
Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N (2020) Lignocellulolytic enzymes in biotechnological and industrial processes: a review. Sustainability 12(18):7282
Janusz G, Pawlik A, Sulej J, Świderska-Burek U, Jarosz-Wilkolazka A, Paszczyński A (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41(6):941–962. https://doi.org/10.1093/femsre/fux049
Chauhan PS, Jha B (2018) Pilot scale production of extracellular thermo-alkali stable laccase from Pseudomonas sp. S2 using agro waste and its application in organophosphorous pesticides degradation. J Chem Technol Biotechnol 93(4):1022–1030
Chandra R, Singh R, Yadav S (2012) Effect of bacterial inoculum ratio in mixed culture for decolourization and detoxification of pulp paper mill effluent. J Chem Technol Biotechnol 87(3):436–444
Niladevi KN, Prema P (2005) Mangrove actinomycetes as the source of ligninolytic enzymes. Actinomycetologica 19(2):40–47
Woo HL, Hazen TC, Simmons BA, DeAngelis KM (2014) Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst Appl Microbiol 37(1):60–67
Katayama Y, Nishikawa S, Murayama A, Yamasaki M, Morohoshi N, Haraguchi T (1988) The metabolism of biphenyl structures in lignin by the soil bacterium (Pseudomonas paucimobilis SYK-6). FEBS Lett 233(1):129–133
Suman SK, Dhawaria M, Tripathi D, Raturi V, Adhikari DK, Kanaujia PK (2016) Investigation of lignin biodegradation by Trabulsiella sp. isolated from termite gut. Int Biodeterior Biodegradation 112:12–17
Pelmont J, Tournesac C, Mliki A, Barrelle M, Beguin C (1989) A new bacterial alcohol dehydrogenase active on degraded lignin and several low molecular weight aromatic compounds. FEMS Microbiol Lett 57(1):109–113
Song YJ (2009) Characterization of aromatic hydrocarbon-degrading bacteria isolated from pine litter. Microbiol Biotechnol Lett 37(4):333–339
Rahman NHA, Abd Aziz S, Hassan MA (2013) Production of ligninolytic enzymes by newly isolated bacteria from palm oil plantation soils. BioResources 8(4):6136–6150
Asina F, Brzonova I, Voeller K, Kozliak E, Kubátová A, Yao B, Ji Y (2016) Biodegradation of lignin by fungi, bacteria and laccases. Bioresour Technol 220:414–424
Bušić A, Morzak G, Belskaya H, Šantek I (2018) Bioethanol production from renewable raw materials and its separation and purification : a review. Food Technol Biotechnol. https://doi.org/10.17113/ftb.56.03.18.5546
Kracher D, Ludwig R (2016) Cellobiose dehydrogenase: an essential enzyme for lignocellulose degradation in nature—a review. Bodenkultur 67:145–163
Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B (2018) Lignocellulose degradation: an overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci 18(11):768–778. https://doi.org/10.1002/elsc.201800039
Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B (2013) Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels 6(1):41. https://doi.org/10.1186/1754-6834-6-41
Rytioja J, Hildén K, Yuzon J, Hatakka A, de Vries RP, Mäkelä MR (2014) Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol Mol Biol Rev 78(4):614–649. https://doi.org/10.1128/MMBR.00035-14
Javed Z, Tripathi GD, Mishra M, Dashora K (2021) Actinomycetes—the microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal Agric Biotechnol 31:101893
Kirby R (2005) Actinomycetes and lignin degradation. Adv Appl Microbiol 58:125–168
Shivlata L, Satyanarayana T (2015) Thermophilic and alkaliphilic Actinobacteria: biology and potential applications. Front Microbiol 6:1014. https://doi.org/10.3389/fmicb.2015.01014
Ravindran R, Jaiswal AK (2016) Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering. https://doi.org/10.3390/bioengineering3040030
Kameshwar AKS, Qin W (2017) Qualitative and quantitative methods for isolation and characterization of lignin-modifying enzymes secreted by microorganisms. BioEnergy Res 10(1):248–266
Sim S-L, He T, Tscheliessnig A, Mueller M, Tan RBH, Jungbauer A (2012) Protein precipitation by polyethylene glycol: a generalized model based on hydrodynamic radius. J Biotechnol 157(2):315–319
Boeris V, Balce I, Vennapusa RR, Rodríguez MA, Picó G, Lahore MF (2012) Production, recovery and purification of a recombinant β-galactosidase by expanded bed anion exchange adsorption. J Chromatogr B 900:32–37
Bhattacharjee S et al (2012) Formation of high-capacity protein-adsorbing membranes through simple adsorption of poly (acrylic acid)-containing films at low pH. Langmuir 28(17):6885–6892
Nguyen HH, Kim M (2017) An overview of techniques in enzyme immobilization. Appl Sci Converg Technol 26(6):157–163. https://doi.org/10.5757/asct.2017.26.6.157
Naresh V, Lee N (2021) A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors (Switzerland) 21(4):1–35. https://doi.org/10.3390/s21041109
Coskun O (2016) Separation techniques: chromatography. North Clin Istanbul 3(2):156–160. https://doi.org/10.14744/nci.2016.32757
Ramos OL, Malcata FX (2017) Food-grade enzymes ☆. In: Moo-Young E III (ed) Comprehensive biotechnology. Pergamon, Oxford, pp 587–603
Masoodi KZ, Lone SM, Rasool RS (2021) Chapter 23—study of principle of centrifugation. In: Masoodi KZ, Lone SM, Rasool B (eds) Advanced methods in molecular biology and biotechnology. Academic Press, pp 133–137
Singhania RR, Patel AK, Soccol CR, Pandey A (2009) Recent advances in solid-state fermentation. Biochem Eng J 44(1):13–18
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 81:146–161
Pandey A (2003) Solid-state fermentation. Biochem Eng J 13(2–3):81–84
Steudler S, Böhmer U, Weber J, Bley T (2015) Biomass measurement by flow cytometry during solid-state fermentation of basidiomycetes. Cytom Part A 87(2):176–188
Lizardi-Jiménez MA, Hernández-Martínez R (2017) Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech 7(1):44
Maulini-Duran C, Abraham J, Rodríguez-Pérez S, Cerda A, Jiménez-Peñalver P, Gea T, Sánchez A (2015) Gaseous emissions during the solid state fermentation of different wastes for enzyme production at pilot scale. Bioresour Technol 179:211–218
Cheah WY et al (2020) Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J 7(1):1115
Bhatia S (2018) Technologies and procedures involved in enzyme production. Introduction to pharmaceutical biotechnology, vol 2. IOP Publishing, pp 2–42
Harrison STL (2011). In: Moo-Young E II (ed) Cell disruption”. Academic Press, Burlington, pp 619–640
Lee CH (2017) A simple outline of methods for protein isolation and purification. Endocrinol Metab (Seoul, Korea) 32(1):18–22. https://doi.org/10.3803/EnM.2017.32.1.18
Alessi AM et al (2018) Defining functional diversity for lignocellulose degradation in a microbial community using multi-omics studies. Biotechnol Biofuels 11(1):1–16
Schmeisser C, Steele H, Streit WR (2007) Metagenomics, biotechnology with non-culturable microbes. Appl Microbiol Biotechnol 75(5):955–962
Silva JP, Ticona ARP, Hamann PRV, Quirino BF, Noronha EF (2021) Deconstruction of lignin: from enzymes to microorganisms. Molecules 26(8):2299
Amer B, Baidoo EEK (2021) Omics-driven biotechnology for industrial applications. Front Bioeng Biotechnol 9:30
Mello BL et al (2017) Targeted metatranscriptomics of compost-derived consortia reveals a GH11 exerting an unusual exo-1,4-β-xylanase activity. Biotechnol Biofuels 10:254. https://doi.org/10.1186/s13068-017-0944-4
Parro V, Moreno-Paz M, González-Toril E (2007) Analysis of environmental transcriptomes by DNA microarrays. Environ Microbiol 9(2):453–464
Aguiar-Pulido V, Huang W, Suarez-Ulloa V, Cickovski T, Mathee K, Narasimhan G (2016) Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis: supplementary issue: bioinformatics methods and applications for big metagenomics data. Evol Bioinforma 12:EBO-S36436
Yadav RK et al (2014) Construction of sized eukaryotic cDNA libraries using low input of total environmental metatranscriptomic RNA. BMC Biotechnol 14(1):1–6
An J et al (2020) Recent advances in enzyme-nanostructure biocatalysts with enhanced activity. Catalysts 10(3):338
Shin HY, Park TJ, Il Kim M (2015) Recent research trends and future prospects in nanozymes. J Nanomater. https://doi.org/10.1155/2015/756278
Korschelt K, Tahir MN, Tremel W (2018) A step into the future: applications of nanoparticle enzyme mimics. Chemistry 24(39):9703–9713. https://doi.org/10.1002/chem.201800384
Chapman R, Stenzel MH (2019) All wrapped up: stabilization of enzymes within single enzyme nanoparticles. J Am Chem Soc 141(7):2754–2769. https://doi.org/10.1021/jacs.8b10338
Scott D, Toney M, Muzikár M (2008) Harnessing the mechanism of glutathione reductase for synthesis of active site bound metallic nanoparticles and electrical connection to electrodes. J Am Chem Soc 130(3):865–874. https://doi.org/10.1021/ja074660g
van Etten MPC, Zijlstra B, Hensen EJM, Filot IAW (2021) Enumerating active sites on metal nanoparticles: understanding the size dependence of cobalt particles for CO dissociation. ACS Catal 11(14):8484–8492. https://doi.org/10.1021/acscatal.1c00651
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42(14):6060–6093. https://doi.org/10.1039/c3cs35486e
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KD: Corresponding author, Conceptualizing and finalizing the draft manuscript. MG and RS: statistical analysis and diagrams. ZJ and GDT: molecular biotechnology information processing. MM, AB and SS: review, proof reading and content processing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal or human studies were carried out by the authors. Under authors’ observation, collection of dung was done by a trained person from the gaushala.
Consent for publication
On behalf of all the authors, I am authorized to give the consent for publishing this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dashora, K., Gattupalli, M., Javed, Z. et al. Leveraging multiomics approaches for producing lignocellulose degrading enzymes. Cell. Mol. Life Sci. 79, 132 (2022). https://doi.org/10.1007/s00018-022-04176-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04176-7