Abstract
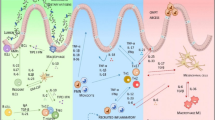
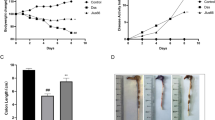
Sam68 is an RNA-binding protein with an adaptor role in signal transduction. Our previous work identified critical proinflammatory and apoptotic functions for Sam68, downstream of the TNF/TNFR1 and TLR2/3/4 pathways. Recent studies have shown elevated Sam68 in inflamed tissues from rheumatoid arthritis and ulcerative colitis (UC) patients, suggesting that Sam68 contributes to chronic inflammatory diseases. Here, we hypothesized that deletion of Sam68 is protective against experimental colitis in vivo, via reductions in TNF-associated inflammatory signaling. We used Sam68 knockout (KO) mice to study the role of Sam68 in experimental colitis, including its contributions to TNF-induced inflammatory gene expression in three-dimensional intestinal organoid cultures. We also studied the expression of Sam68 and inflammatory genes in colon tissues of UC patients. Sam68 KO mice treated with an acute course of DSS exhibited significantly less weight loss and histopathological inflammation compared to wild-type controls, suggesting that Sam68 contributes to experimental colitis. Bone marrow transplants showed no pathologic role for hematopoietic cell-specific Sam68, suggesting that non-hematopoietic Sam68 drives intestinal inflammation. Gene expression analyses showed that Sam68 deficiency reduced the expression of proinflammatory genes in colon tissues from DSS-treated mice, as well as TNF-treated three-dimensional colonic organoids. We also found that inflammatory genes, such as TNF, CCR2, CSF2, IL33 and CXCL10, as well as Sam68 protein, were upregulated in inflamed colon tissues of UC patients. This report identifies Sam68 as an important inflammatory driver in response to intestinal epithelial damage, suggesting that targeting Sam68 may hold promise to treat UC patients.







Similar content being viewed by others
Availability of data and material
All of the data underlying this article are available in the article and in its online supplementary data. Materials such as reagents will be available upon request.
Code availability
Not applicable.
References
de Souza HSP, Fiocchi C, Iliopoulos D (2017) The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 14(12):739–749
Goodman WA, Erkkila IP, Pizarro TT (2020) Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17:740–754
Neurath MF (2017) Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 14(5):269–278
Nunez FP et al (2020) Treat-to-target approach in the management of inflammatory Bowel disease. Gastroenterol Hepatol 44:31–319
D’Haens G (2007) Risks and benefits of biologic therapy for inflammatory bowel diseases. Gut 56(5):725–732
Leppkes M et al (2014) Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. Int Immunol 26(9):509–515
Lu Y et al (2018) Toll-like receptors and inflammatory bowel disease. Front Immunol 9:72
Papadakis KA, Targan SR (2000) Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51:289–298
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104(4):487–501
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214(2):149–160
Lukong KE, Richard S (2003) Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta 1653(2):73–86
Ramakrishnan P, Baltimore D (2011) Sam68 is required for both NF-kappaB activation and apoptosis signaling by the TNF receptor. Mol Cell 43(2):167–179
Tomalka JA, de Jesus TJ, Ramakrishnan P (2017) Sam68 is a regulator of Toll-like receptor signaling. Cell Mol Immunol 14(1):107–117
Fusaki N et al (1997) Interaction between Sam68 and Src family tyrosine kinases, Fyn and Lck, in T cell receptor signaling. J Biol Chem 272(10):6214–6219
Frisone P et al (2015) SAM68: signal transduction and RNA metabolism in human cancer. Biomed Res Int 2015:528954
Busa R et al (2007) The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene 26(30):4372–4382
Fu K et al (2016) Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-kappaB activation. Elife 5:e15018
Qian J et al (2016) Sam68 modulates apoptosis of intestinal epithelial cells via mediating NF-kappaB activation in ulcerative colitis. Mol Immunol 75:48–59
Sun W et al (2018) Sam68 promotes invasion, migration, and proliferation of fibroblast-like synoviocytes by enhancing the NF-kappaB/P65 pathway in rheumatoid arthritis. Inflammation 41(5):1661–1670
Sato T et al (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469(7330):415–418
de Jesus TJ, Ramakrishnan P (2020) NF-kappaB c-Rel dictates the inflammatory threshold by acting as a transcriptional repressor. iScience 23(3):100876
Fu K et al (2013) Sam68 modulates the promoter specificity of NF-kappaB and mediates expression of CD25 in activated T cells. Nat Commun 4:1909
Perez-Perez A et al (2016) Sam68 mediates the activation of insulin and leptin signalling in breast cancer cells. PLoS ONE 11(7):e0158218
Sanchez-Margalet V, Najib S (2001) Sam68 is a docking protein linking GAP and PI3K in insulin receptor signaling. Mol Cell Endocrinol 183(1–2):113–121
Goodman WA et al (2018) Estrogen receptor alpha loss-of-function protects female mice from DSS-induced experimental colitis. Cell Mol Gastroenterol Hepatol 5(4):630-633 e1
Chassaing B et al (2014) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104:15–25
Qiao A et al (2021) Ablation of Sam68 in adult mice increases thermogenesis and energy expenditure. FASEB J 35(8):e21772
Scaldaferri F et al (2007) Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest 117(7):1951–1960
Scaldaferri F et al (2009) VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 136(2):585–95 e5
Okayasu I et al (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98(3):694–702
Jabado N et al (1998) Sam68 association with p120GAP in CD4+ T cells is dependent on CD4 molecule expression. J Immunol 161(6):2798–2803
Gilbert C et al (2001) Evidence for a role for SAM68 in the responses of human neutrophils to ligation of CD32 and to monosodium urate crystals. J Immunol 166(7):4664–4671
Grossmann J et al (1998) New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol 153(1):53–62
Ungaro R et al (2017) Ulcerative colitis. Lancet 389(10080):1756–1770
Jarnerot G et al (2005) Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 128(7):1805–1811
Reinisch W et al (2011) Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 60(6):780–787
Rutgeerts P et al (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353(23):2462–2476
Sandborn WJ et al (2014) Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146(1):85–95 (quiz e14-5)
Tomalka JA, de Jesus TJ, Ramakrishnan P (2016) Sam68 is a regulator of Toll-like receptor signaling. Cell Mol Immunol
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621
Fuss IJ et al (1996) Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 157(3):1261–1270
Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3(7):390–407
Shivaji UN et al (2019) Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther 49(6):664–680
Vulliemoz M et al (2020) TNF-Alpha blockers in inflammatory bowel diseases: practical recommendations and a user’s guide: an update. Digestion 101(Suppl 1):16–26
Lukong KE et al (2005) Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem 280(46):38639–38647
Paronetto MP et al (2007) The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol 176(7):929–939
Resnick RJ et al (1997) Phosphorylation of the Src substrate Sam68 by Cdc2 during mitosis. Oncogene 15(11):1247–1253
Monteleone G et al (2005) Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 129(5):1420–1429
Hu D et al (2015) Interplay between arginine methylation and ubiquitylation regulates KLF4-mediated genome stability and carcinogenesis. Nat Commun 6:8419
Lorton BM, Shechter D (2019) Cellular consequences of arginine methylation. Cell Mol Life Sci 76(15):2933–2956
Babic I, Jakymiw A, Fujita DJ (2004) The RNA binding protein Sam68 is acetylated in tumor cell lines, and its acetylation correlates with enhanced RNA binding activity. Oncogene 23(21):3781–3789
Cote J et al (2003) Sam68 RNA binding protein is an in vivo substrate for protein arginine N-methyltransferase 1. Mol Biol Cell 14(1):274–287
Hayashi S et al (2017) PI3K p85alpha subunit-deficient macrophages protect mice from acute colitis due to the enhancement of IL-10 production. Sci Rep 7(1):6187
Kim JK et al (2016) Grim19 attenuates DSS induced colitis in an animal model. PLoS ONE 11(6):e0155853
Sun M et al (2017) Hypoxia inducible factor-1alpha-induced interleukin-33 expression in intestinal epithelia contributes to mucosal homeostasis in inflammatory bowel disease. Clin Exp Immunol 187(3):428–440
Paronetto MP et al (2009) Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J Cell Biol 185(2):235–249
Richard S et al (2005) Ablation of the Sam68 RNA binding protein protects mice from age-related bone loss. PLoS Genet 1(6):e74
Hu B et al (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA 107(50):21635–21640
Mantovani A et al (2008) Cancer-related inflammation. Nature 454(7203):436–444
Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48(4):526–535
Jess T, Rungoe C, Peyrin-Biroulet L (2012) Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 10(6):639–645
Lutgens MW et al (2013) Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 19(4):789–799
Wang Y et al (2017) Expression of Sam68 correlates with cell proliferation and survival in epithelial ovarian cancer. Reprod Sci 24(1):97–108
Raval G, Mehta P (2010) TNF-alpha inhibitors: are they carcinogenic? Drug Healthc Patient Saf 2:241–247
Acknowledgements
The authors thank Dr. Stephane Richard, McGill University for providing the Sam68 KO mice. We thank the Cleveland Digestive Disease Research Core Center (DDRCC) for support, particularly Drs. Fabio Cominelli, M.D., Ph.D. (Center Director and Mouse Models Core Director), Dr. Theresa Pizarro, Ph.D. (Associate Center Director and Histology/Imaging Core Director), and Dr. Claudio Fiocchi, M.D. (Associate Center Director and Biorepository Core Director). We also thank Dr. Wei Xin, M.D., Ph.D., board-certified DDRCC Pathologist, for his expert analysis of DSS-treated colon tissues.
Funding
This work was supported by supported by the National Institute of Allergy and Infectious Diseases at National Institute of Health grants R01 AI116730 and R21 AI144264, and National Cancer Institute at National Institute of Health grant R21 CA246194, to P.R. Research reported in this publication was also supported by the National Institute Of Diabetes And Digestive And Kidney Diseases at the National Institutes of Health under Award Number P30DK097948 that supported Cleveland Digestive Disease Research Core Center Pilot/Feasibility Award to P.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. W.A.G. was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases at the National Institutes of Health grants R01 DK128143, K01 DK105138, R03 DK123579, and Crohn’s & Colitis Foundation Senior Research Award #635911. A.R.L was supported by the National Institute of Health NIH/NEI T32 pre-doctoral training grant T32EY007157.
Author information
Authors and Affiliations
Contributions
WAG performed experiments, analyzed data, and wrote the manuscript. SB performed experiments and analyzed data. ARL, FDSR and TM performed experiments. PR conceived of the study, performed experiments, analyzed data and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
PR has a patent on the use of Sam68 for modulating signaling through the TNF receptor (US8598137B2). Other authors have declared that no conflict of interest exists.
Ethics approval
All mouse studies were approved under CWRU IACUC protocol 2013-0134. All human tissue samples used in this study were slated to be discarded samples that were deidentified and procured through the Cleveland Digestive Disease Research Biorepositoy Core facility with non-human subject research IRB approval from University Hospitals, IRB number NHR-16-103 and STUDY20210825.
Consent to participate
Not applicable. No human subjects were recruited for this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goodman, W.A., Basavarajappa, S.C., Liu, A.R. et al. Sam68 contributes to intestinal inflammation in experimental and human colitis. Cell. Mol. Life Sci. 78, 7635–7648 (2021). https://doi.org/10.1007/s00018-021-03976-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03976-7