Abstract
Background
Chronic pancreatitis (CP) is a progressive disease characterized by pancreatic fibrosis for which effective treatment options are lacking. Mesenchymal stem cells (MSCs) have shown potential for fibrosis treatment but face limitations in clinical application. The high-mobility group box 1 (HMGB1) fragment mobilizes MSCs from bone marrow into the blood and has emerged as a promising therapeutic agent for tissue regeneration in various pathological conditions. The aim of this study was to investigate the potential therapeutic effects of systemic administration of the HMGB1 fragment in a mouse model of CP.
Methods
A caerulein-induced CP mouse model was used, and the HMGB1 fragment was administered by tail vein injection. Parameters such as body weight, pancreatic tissue damage, fibrosis, inflammatory cytokine expression, and collagen-related gene expression were evaluated using various assays, including immunohistochemistry, real-time PCR, serum analysis, and single-cell transcriptome analysis. And the migration of MSCs to the pancreas was evaluated using the parabiosis model.
Results
Administration of the HMGB1 fragment was associated with significant improvements in pancreatic tissue damage and fibrosis. It suppressed the expression of inflammatory cytokines and activated platelet-derived growth factor receptor-α+ MSCs, leading to their accumulation in the pancreas. The HMGB1 fragment also shifted gene expression patterns associated with pancreatic fibrosis toward those of the normal pancreas. Systemic administration of the HMGB1 fragment demonstrated therapeutic efficacy in attenuating pancreatic tissue damage and fibrosis in a CP mouse model.
Conclusion
These findings highlight the potential of the HMGB1 fragment as a therapeutic target for the treatment of CP.





Similar content being viewed by others
Data availability
All sequencing data used in this study were uploaded to GEO (https://www.ncbi.nlm.nih.gov/geo/; accession number: GSE247430). Other data are included in the manuscript and/or supporting information.
Abbreviations
- AP:
-
Acute pancreatitis
- α-SMA:
-
α-Smooth muscle actin
- CTGF:
-
Connective tissue growth factor
- CP:
-
Chronic pancreatitis
- CXCR4:
-
C-X-C chemokine receptor 4
- DEG:
-
Differentially expressed gene
- FBS:
-
Fetal bovine serum
- GFP:
-
Green fluorescent protein
- H&E:
-
Hematoxylin and eosin
- HMGB1:
-
High-mobility group box 1
- LDH:
-
Lactate dehydrogenase
- MSC:
-
Mesenchymal stem cell
- PDGFRα:
-
Platelet-derived growth factor receptor-α
- PSC:
-
Pancreatic stellate cell
- SDF-1:
-
Stromal cell-derived factor 1
- UMAP:
-
Uniform manifold approximation and projection
References
Beyer G, Habtezion A, Werner J, et al. Chronic pancreatitis. Lancet. 2020;396:499–512.
Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106:2192–9.
Hirota M, Shimosegawa T, Masamune A, et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology. 2012;12:79–84.
Olesen SS, Mortensen LH, Zinck E, et al. Time trends in incidence and prevalence of chronic pancreatitis: a 25-year population-based nationwide study. United European Gastroenterol J. 2021;9:82–90.
Falconi M, Bassi C, Casetti L, et al. Long-term results of Frey’s procedure for chronic pancreatitis: a longitudinal prospective study on 40 patients. J Gastrointest Surg. 2006;10:504–10.
Shimizu K, Ito T, Irisawa A, et al. Evidence-based clinical practice guidelines for chronic pancreatitis 2021. J Gastroenterol. 2022;57:709–24.
Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989–94.
Zeng XP, Wang LJ, Guo HL, et al. Dasatinib ameliorates chronic pancreatitis induced by caerulein via anti-fibrotic and anti-inflammatory mechanism. Pharmacol Res. 2019;147: 104357.
Nadella S, Ciofoaia V, Cao H, et al. Cholecystokinin receptor antagonist therapy decreases inflammation and fibrosis in chronic pancreatitis. Dig Dis Sci. 2020;65:1376–84.
Tamura T, Kodama T, Sato K, et al. Dysregulation of PI3K and Hippo signaling pathways synergistically induces chronic pancreatitis via CTGF upregulation. J Clin Invest. 2021;131.
Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47.
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7.
Oh EJ, Lee HW, Kalimuthu S, et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J Control Release. 2018;279:79–88.
Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8:271–84.
Zhou C-H, Li M-L, Qin A-L, et al. Reduction of fibrosis in dibutyltin dichloride-induced chronic pancreatitis using rat umbilical mesenchymal stem cells from Wharton’s jelly. Pancreas. 2013;42:1291–302.
Kawakubo K, Ohnishi S, Fujita H, et al. Effect of fetal membrane-derived mesenchymal stem cell transplantation in rats with acute and chronic pancreatitis. Pancreas. 2016;45:707–13.
Xiao Ling K, Peng L, Jian Feng Z, et al. Stromal derived factor-1/CXCR4 axis involved in bone marrow mesenchymal stem cells recruitment to injured liver. Stem Cells Int. 2016;2016:8906945.
Yang YK, Ogando CR, Wang See C, et al. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131.
Watanabe T, Sadakane Y, Yagama N, et al. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce IL-33-dependent chronic pancreatitis. Mucosal Immunol. 2016;9:1234–49.
Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283–98.
Tamai K, Yamazaki T, Chino T, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A. 2011;108:6609–14.
Aikawa E, Fujita R, Kikuchi Y, et al. Systemic high-mobility group box 1 administration suppresses skin inflammation by inducing an accumulation of PDGFRα(+) mesenchymal cells from bone marrow. Sci Rep. 2015;5:11008.
Goto T, Miyagawa S, Tamai K, et al. High-mobility group box 1 fragment suppresses adverse post-infarction remodeling by recruiting PDGFRalpha-positive bone marrow cells. PLoS ONE. 2020;15: e0230392.
Nojiri S, Tsuchiya A, Natsui K, et al. Synthesized HMGB1 peptide attenuates liver inflammation and suppresses fibrosis in mice. Inflamm Regen. 2021;41:28.
Yamada T, Araki H, Watabe K, et al. Adiponectin deficiency enhanced the severity of cerulein-induced chronic pancreatitis in mice. J Gastroenterol. 2010;45:742–9.
Gong J, Meng HB, Hua J, et al. The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol Med Rep. 2014;9:1575–82.
Miura A, Shimbo T, Kitayama T, et al. Contribution of PDGFRα lineage cells in adult mouse hematopoiesis. Biochem Biophys Res Commun. 2021;534:186–92.
Ishibashi K, Ikegami K, Shimbo T, et al. Single-cell transcriptome analysis reveals cellular heterogeneity in mouse intra- and extra articular ligaments. Commun Biol. 2022;5:1233.
https://jp.support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html.
Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Hao Y, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573-87.e29.
Yang C, Liu ZL, Wang J, et al. Parabiosis modeling: protocol, application and perspectives. Zool Res. 2021;42:253–61.
Klauss S, Schorn S, Teller S, et al. Genetically induced vs. classical animal models of chronic pancreatitis: a critical comparison. FASEB J. 2018:fj201800241RR.
Tomaszewska E, Świątkiewicz M, Muszyński S, et al. Repetitive cerulein-induced chronic pancreatitis in growing pigs—a pilot study. Int J Mol Sci. 2023;24.
Han X, Li B, Bao J, et al. Endoplasmic reticulum stress promoted acinar cell necroptosis in acute pancreatitis through cathepsinB-mediated AP-1 activation. Front Immunol. 2022;13: 968639.
Yang L, Xie M, Yang M, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014;5:4436.
Baxevanis AD, Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 1995;23:1604–13.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42.
Arriaga-Pizano L, Boscó-Gárate I, Martínez-Ordaz JL, et al. High serum levels of high-mobility group box 1 (HMGB1) and low levels of heat shock protein 70 (Hsp70) are associated with poor prognosis in patients with acute pancreatitis. Arch Med Res. 2018;49:504–11.
Wang YS, Li YY, Wang LH, et al. Tanshinone IIA attenuates chronic pancreatitis-induced pain in rats via downregulation of HMGB1 and TRL4 expression in the spinal cord. Pain Physician. 2015;18:E615–28.
Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–107.
Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol. 2015;21:1424–35.
Ishii Y, Tsuchiya A, Natsui K, et al. Synthesized HMGB1 peptide prevents the progression of inflammation, steatosis, fibrosis, and tumor occurrence in a non-alcoholic steatohepatitis mouse model. Hepatol Res. 2022;52:985–97.
Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48.
Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8.
Aghdassi AA, Mayerle J, Christochowitz S, et al. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26.
Acknowledgements
Shogo Kobayashi received fees from AstraZeneca and Taiho for the time and energy spent attending a meeting or giving a presentation or advice. Katsuto Tamai reports financial support was provided by Stem RIM Inc. Takashi Shimbo received a research grant from Stem RIM Inc. Katsuto Tamai has patent issued to Osaka University, StemRIM.
The authors gratefully acknowledge all members of the Department of Gastroenterological Surgery and the Department of Stem Cell Therapy Science at Osaka University, and all the staff in StemRIM Institute of Regeneration-Inducing Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2024_2112_MOESM1_ESM.tif
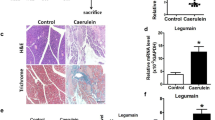
Supplementary file1 HMGB1 assay of serum from normal and CP model mice. No differences were found between serum HMGB1 in normal mice and serum HMGB1 in CP model mice (n = 5 per group). *P < 0.05. CP chronic pancreatitis, HMGB1 high-mobility group box 1 (TIF 859 KB)
535_2024_2112_MOESM2_ESM.tif
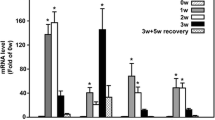
Supplementary file2 RT-PCR analysis of mRNA expression of mediator of fibrosis in control and HMGB1 pancreas tissue in the4th week (n = 5 per group). †P < 0.10 compared with the control group. *P < 0.05 compared with the control group. **P < 0.01 compared with the control group. Mmp2 matrix metalloproteinase-2, Mmp9 matrix metalloproteinase-9, Tgfb1 transforming growth factor beta 1, RT-PCR reverse transcription polymerase chain reaction (TIF 917 KB)
535_2024_2112_MOESM3_ESM.tif
Supplementary file3 Serum analysis in the control and HMGB1 groups. (a–c) Serum LDH, amylase, and lipase levels in the control and HMGB1 groups in the fourth, sixth, and eighth weeks (n = 5 per group). *P < 0.05 compared with the control group. **P < 0.01 compared with the control group. HMGB1 high-mobility group box 1, LDH lactate dehydrogenas (TIF 1486 KB)
535_2024_2112_MOESM4_ESM.tif
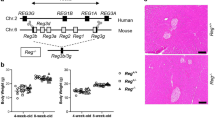
Supplementary file4 Effect of HMGB1 fragment administration in fully developed CP model mice. (a) Schematic of HMGB1 fragment administration for the CP model mice. Caerulein was administered to C57BL/6 male mice intraperitoneally six times a day, three times a week for 8 weeks. Four weeks after the initiation of caerulein administration, HMGB1 fragments or normal saline was administered intravenously (i.v.) three times a week for 4 weeks. (b) Body weight change in the control and HMGB1 groups (n = 5 per group). (c) Percent ratio of pancreas weight to body weight in the control and HMGB1 groups (n = 5 per group). *P < 0.05 compared with the control group. **P < 0.01 compared with the control group. (d) Representative H&E staining of pancreas tissue sections from the control (upper row) and HMGB1 (lower row) groups in the sixth and eighth weeks. Scale bars, 100 μm. (e) RT-PCR analysis of mRNA expression of inflammatory cytokines in control and HMGB1 pancreas tissue in the eighth week (n = 5 per group). *P < 0.05 compared with the control group. **P < 0.01 compared with the control group. (f) Representative Sirius Red-stained tissues and stained areas in the control and HMGB1 groups (n = 5 per group). *P < 0.05 compared with the control group. Scale bars, 100 μm. (g) Quantification of hydroxyproline levels at the sixth and eighth weeks in the control and HMGB1 groups (n = 5 per group). *P < 0.05 compared with the control group. CP chronic pancreatitis, H&E hematoxylin and eosin, HMGB1 high-mobility group box 1, HYP hydroxyproline, RT-PCR reverse transcription polymerase chain reaction (TIF 49852 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hokkoku, D., Sasaki, K., Kobayashi, S. et al. High-mobility group box 1 fragment ameliorates chronic pancreatitis induced by caerulein in mice. J Gastroenterol (2024). https://doi.org/10.1007/s00535-024-02112-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00535-024-02112-z