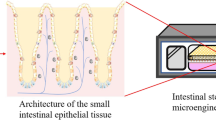
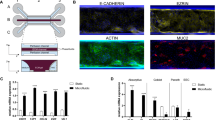
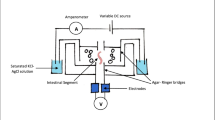
Abstract
Inflammatory Bowel Disease (IBD) affects a substantial global population and is characterized by chronic inflammation primarily in the gastrointestinal tract. The pathophysiology of IBD is intricate and involves a combination of genetic, environmental, and immune-related factors. This article aims to discuss the current understanding of IBD pathophysiology, focusing on the roles of genetics, environmental triggers, and the immune system in disease development and progression. In addition, the review covers the existing treatment options for IBD, including medical and surgical interventions while addressing the challenges associated with managing this chronic condition. Furthermore, the article explores the utility of disease modeling techniques, such as animal models, in vitro organoid models, and microfluidic organ-on-a-chip devices, for studying IBD pathophysiology and evaluating potential therapeutic interventions. It is important to note that conventional two-dimensional (2D) cell cultures are limited in capturing essential physical and biochemical cues, despite displaying lineage-specific differences. The translation of data from animal models to human physiology in major systems undermines the reliability of the generated data. To address these limitations, one noteworthy solution is the utilization of microfluidic-based organ-on-a-chip devices, which can effectively mimic organ functionality. These devices are developed based on principles of microfluidics, materials science, and cell biology. Overall, this comprehensive review provides insights into the pathophysiology of IBD, current treatment options, and disease modeling approaches, including emerging technologies, such as organoids and organ-on-a-chip. These advancements offer promising avenues for enhancing our understanding of IBD and developing more efficient therapies for this debilitating condition.





Similar content being viewed by others
References
Joshi, A., Soni, A., Acharya, S.: In vitro models and ex vivo systems used in inflammatory bowel disease. In Vitro Models 1(3), 213–227 (2022)
Guan, Q.: A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019, 7247238 (2019)
Head, K.A., Jurenka, J.S.: Inflammatory bowel disease Part 1: ulcerative colitis–pathophysiology and conventional and alternative treatment options. Altern Med Rev 8(3), 247–283 (2003)
Head, K., Jurenka, J.S.: Inflammatory bowel disease. Part II: Crohn’s disease–pathophysiology and conventional and alternative treatment options. Altern Med Rev 9(4), 360–401 (2004)
Mak, W.Y., et al.: The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol 35(3), 380–389 (2020)
Steinway, S.N., et al.: Human microphysiological models of intestinal tissue and gut microbiome. Front Bioeng Biotechnol 8, 725 (2020)
Goyal, N., et al.: Animal models of inflammatory bowel disease: a review. Inflammopharmacology 22(4), 219–233 (2014)
Nguyen, L.H., et al.: Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol 5(11), 986–995 (2020)
Nicolaides, S., et al.: The impact of tobacco smoking on treatment choice and efficacy in inflammatory bowel disease. Intest Res 19(2), 158–170 (2021)
Chiba, M., Nakane, K., Komatsu, M.: Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J 23, 18–107 (2019)
Atreya, R., Neurath, M.F.: IBD pathogenesis in 2014: molecular pathways controlling barrier function in IBD. Nat Rev Gastroenterol Hepatol 12(2), 67–68 (2015)
Beaurivage, C., et al., Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int J Mol Sci, 2019. 20(22).
Mizoguchi, A.: Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci 105, 263–320 (2012)
Mokry, M., et al.: Many inflammatory bowel disease risk loci include regions that regulate gene expression in immune cells and the intestinal epithelium. Gastroenterology 146(4), 1040–1047 (2014)
Prinz, F., Schlange, T., Asadullah, K.: Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10(9), 712 (2011)
Dawson, A., et al.: A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow. Biomicrofluidics 10(6), 064101 (2016)
Edmondson, R., et al.: Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12(4), 207–218 (2014)
Kim, H.J., et al.: Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA 113(1), E7-15 (2016)
Halldorsson, S., et al.: Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 63, 218–231 (2015)
Carter, M.J., et al.: Guidelines for the management of inflammatory bowel disease in adults. Gut 535(Suppl 5), VI–16 (2004)
Mirkov, M.U., Verstockt, B., Cleynen, I.: Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol Hepatol 2(3), 224–234 (2017)
Peters, L.A., et al.: A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat Genet 49(10), 1437–1449 (2017)
Ogura, Y., et al.: A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411(6837), 603–606 (2001)
Yamamoto, S., Ma, X.: Role of Nod2 in the development of Crohn’s disease. Microbes Infect 11(12), 912–918 (2009)
Inohara, N., et al.: Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 278(8), 5509–5512 (2003)
Strober, W., et al.: The molecular basis of NOD2 susceptibility mutations in Crohn’s disease. Mucosal Immunol 11(01), S5-9 (2008)
Magalhaes, J.G., et al.: What is new with Nods? Curr Opin Immunol 23(1), 29–34 (2011)
Cohen, L.J., et al.: Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 156(8), 2174–2189 (2019)
Kabat, A.M., et al.: The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife 5, e12444 (2016)
Ahern, P.P., et al.: Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33(2), 279–288 (2010)
Sun, R., Hedl, M., Abraham, C.: IL23 induces IL23R recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the IBD-protective IL23R R381Q variant modulates these outcomes. Gut 69(2), 264–273 (2020)
Hibi, T., Ogata, H.: Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol 41(1), 10–16 (2006)
Dolan, K.T. and E.B. Chang, Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res, 2017. 61(1)
Mawdsley, J.E., Rampton, D.S.: The role of psychological stress in inflammatory bowel disease. NeuroImmunoModulation 13(5–6), 327–336 (2006)
Cosnes, J.: Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol 18(3), 481–496 (2004)
Cosnes, J.: What is the link between the use of tobacco and IBD? Inflamm Bowel Dis 14(Suppl 2), S14–S15 (2008)
Birrenbach, T., Bocker, U.: Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis 10(6), 848–859 (2004)
Mawdsley, J.E., Rampton, D.S.: Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 54(10), 1481–1491 (2005)
Thia, K.T., et al.: An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 103(12), 3167–3182 (2008)
Garg, M., et al.: Review article: vitamin D and inflammatory bowel disease–established concepts and future directions. Aliment Pharmacol Ther 36(4), 324–344 (2012)
Maunder, R.G.: Evidence that stress contributes to inflammatory bowel disease: evaluation, synthesis, and future directions. Inflamm Bowel Dis 11(6), 600–608 (2005)
Camara, R.J., et al.: Mood and nonmood components of perceived stress and exacerbation of Crohn’s disease. Inflamm Bowel Dis 17(11), 2358–2365 (2011)
Goodhand, J.R., et al.: Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis 18(7), 1232–1239 (2012)
van Eeden, S.F., et al.: Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med 164(5), 826–830 (2001)
Ananthakrishnan, A.N., et al.: Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis 17(5), 1138–1145 (2011)
Zhang, Y.Z., Li, Y.Y.: Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20(1), 91–99 (2014)
Geremia, A., Jewell, D.P.: The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 6(2), 223–237 (2012)
Medzhitov, R., Janeway, C., Jr.: Innate immunity. N Engl J Med 343(5), 338–344 (2000)
Noguchi, E., et al.: A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol 10(5), 471–479 (2009)
Takatori, H., et al.: Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206(1), 35–41 (2009)
Van der Sluis, M., et al.: Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131(1), 117–129 (2006)
Korn, T., et al.: IL-17 and Th17 Cells. Annu Rev Immunol 27, 485–517 (2009)
Heller, F., et al.: Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129(2), 550–564 (2005)
Wilson, M.S., et al.: Colitis and intestinal inflammation in IL10-/- mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology 140(1), 254–264 (2011)
Sugihara, T., et al.: The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol 160(3), 386–393 (2010)
Sarra, M., et al.: Interferon-gamma-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm Bowel Dis 16(8), 1332–1339 (2010)
Nishida, A., et al.: Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11(1), 1–10 (2018)
Nell, S., Suerbaum, S., Josenhans, C.: The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol 8(8), 564–577 (2010)
Yue, B., et al., Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms, 2019. 7(10).
Tezuka, H., Ohteki, T.: Regulation of intestinal homeostasis by dendritic cells. Immunol Rev 234(1), 247–258 (2010)
Saleh, M., Elson, C.O.: Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity 34(3), 293–302 (2011)
Bruewer, M., et al.: Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171(11), 6164–6172 (2003)
Nusrat, A., Turner, J.R., Madara, J.L.: Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol 279(5), 851–857 (2000)
Ng, S.C., et al.: Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm Bowel Dis 16(10), 1787–1807 (2010)
Ni, J., et al.: Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 14(10), 573–584 (2017)
Melgar, S., Shanahan, F.: Inflammatory bowel disease-from mechanisms to treatment strategies. Autoimmunity 43(7), 463–477 (2010)
Rousseaux, C., et al.: Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med 201(8), 1205–1215 (2005)
Oh-Oka, K., et al.: Induction of colonic regulatory t cells by mesalamine by activating the aryl hydrocarbon receptor. Cell Mol Gastroenterol Hepatol 4(1), 135–151 (2017)
Wang, Y., et al.: Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 4(4), CD000543 (2016)
Bonovas, S., et al.: Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 45(9), 1179–1192 (2017)
Hart, A., et al.: The use of 5-aminosalicylates in Crohn’s disease: a retrospective study using the UK Clinical Practice Research Datalink. Ann Gastroenterol 33(5), 500–507 (2020)
Gjuladin-Hellon, T., et al.: Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn’s disease. Cochrane Database Syst Rev 6(6), CD008414 (2019)
Coward, S., et al.: Comparative effectiveness of mesalamine, sulfasalazine, corticosteroids, and budesonide for the induction of remission in Crohn’s disease: a Bayesian network meta-analysis. Inflamm Bowel Dis 23(3), 461–472 (2017)
Gisbert, J.P., Gonzalez-Lama, Y., Mate, J.: 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis 13(5), 629–638 (2007)
Waljee, A.K., et al.: Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS ONE 11(6), e0158017 (2016)
Choi, C.H., et al.: Second Korean guidelines for the management of ulcerative colitis. Intest Res 15(1), 7–37 (2017)
Ferrante, M., et al.: Physician perspectives on unresolved issues in the use of conventional therapy in Crohn’s disease: results from an international survey and discussion programme. J Crohns Colitis 6(1), 116–131 (2012)
Strehl, C., et al.: Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol 10, 1744 (2019)
An, Y.K.: Common mistakes with steroids. J Gastroenterol Hepatol 36(Suppl 1), 30–31 (2021)
Dubois-Camacho, K., et al.: Glucocorticosteroid therapy in inflammatory bowel diseases: from clinical practice to molecular biology. World J Gastroenterol 23(36), 6628–6638 (2017)
Lee, K.M., et al.: Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res 13(3), 193–207 (2015)
Taylor, K.M., Irving, P.M.: Optimization of conventional therapy in patients with IBD. Nat Rev Gastroenterol Hepatol 8(11), 646–656 (2011)
Winter, J.W., et al.: Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther 25(9), 1069–1077 (2007)
Yang, S.K., et al.: A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 46(9), 1017–1020 (2014)
Matsuoka, K.: NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease. Intest Res 18(3), 275–281 (2020)
Elhag, D.A., et al., Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int J Mol Sci, 2022. 23(13)
Kirchgesner, J., et al.: Impact on life expectancy of withdrawing thiopurines in patients with Crohn’s disease in sustained clinical remission: a lifetime risk-benefit analysis. PLoS ONE 11(6), e0157191 (2016)
Neurath, M.F.: Cytokines in inflammatory bowel disease. Nat Rev Immunol 14(5), 329–342 (2014)
Sandborn, W.J., et al.: Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 137(4), 1250–1260 (2009). (quiz 1520)
Papamichael, K., et al., Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis, 2019. 10: p. 2040622319838443
Sandborn, W.J., et al.: Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146(1), 96-109 e1 (2014)
Adegbola, S.O., et al., Anti-TNF Therapy in Crohn's Disease. Int J Mol Sci, 2018. 19(8)
Rudrapatna, V.A., Velayos, F.: Biosimilars for the treatment of inflammatory bowel disease. Pract Gastroenterol 43(4), 84–91 (2019)
Ben-Horin, S., Kopylov, U., Chowers, Y.: Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 13(1), 24–30 (2014)
Nakase, H., et al.: Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther 46(9), 873–882 (2017)
Wittig, B.M.: Drug evaluation: CNTO-1275, a mAb against IL-12/IL-23p40 for the potential treatment of inflammatory diseases. Curr Opin Investig Drugs 8(11), 947–954 (2007)
Almradi, A., et al.: Clinical trials of IL-12/IL-23 inhibitors in inflammatory bowel disease. BioDrugs 34(6), 713–721 (2020)
Rutgeerts, P., et al.: Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 155(4), 1045–1058 (2018)
Feagan, B.G., et al.: Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 375(20), 1946–1960 (2016)
Papp, K., et al.: Safety surveillance for Ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol 14(7), 706–714 (2015)
Sandborn, W.J., et al.: Efficacy and safety of Mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 158(3), 537-549 e10 (2020)
Feagan, B.G., et al.: Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389(10080), 1699–1709 (2017)
Wong, U., Cross, R.K.: Expert opinion on interleukin-12/23 and interleukin-23 antagonists as potential therapeutic options for the treatment of inflammatory bowel disease. Expert Opin Investig Drugs 28(5), 473–479 (2019)
Jovani, M., Danese, S.: Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr Drug Targets 14(12), 1433–1443 (2013)
Fiorino, G., Gilardi, D., Danese, S.: The clinical potential of etrolizumab in ulcerative colitis: hypes and hopes. Therap Adv Gastroenterol 9(4), 503–512 (2016)
Tang, M.T., et al.: Review article: nonclinical and clinical pharmacology, pharmacokinetics and pharmacodynamics of etrolizumab, an anti-beta7 integrin therapy for inflammatory bowel disease. Aliment Pharmacol Ther 47(11), 1440–1452 (2018)
Feagan, B.G., et al.: Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369(8), 699–710 (2013)
Sands, B.E., et al.: Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 23(1), 97–106 (2017)
Pellet, G., et al.: Efficacy and safety of induction therapy with calcineurin inhibitors in combination with vedolizumab in patients with refractory ulcerative colitis. Clin Gastroenterol Hepatol 17(3), 494–501 (2019)
Sandborn, W.J., et al.: Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv Ther 37(7), 3417–3431 (2020)
Yoshimura, N., et al.: Safety and efficacy of AJM300, an oral antagonist of alpha4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology 149(7), 1775-1783 e2 (2015)
Gubatan, J., et al.: Anti-integrins for the treatment of inflammatory bowel disease: current evidence and perspectives. Clin Exp Gastroenterol 14, 333–342 (2021)
Cai, Z., Wang, S., Li, J.: Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne) 8, 765474 (2021)
Cohen, J.L., et al.: Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum 48(11), 1997–2009 (2005)
Lamb, C.A., et al.: British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68(Suppl 3), s1–s106 (2019)
Fazio, V.W., et al.: Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. A randomized controlled trial. Ann Surg 224(4), 563–571 (1996). (discussion 571–3)
Rutgeerts, P., et al.: Predictability of the postoperative course of Crohn’s disease. Gastroenterology 99(4), 956–963 (1990)
Kumar, M., Garand, M., Al Khodor, S.: Integrating omics for a better understanding of inflammatory bowel disease: a step towards personalized medicine. J Transl Med 17(1), 419 (2019)
Satoh, K., et al.: Severe sepsis caused by bacteria that entered via the intestinal tract: a case of Crohn’s disease in a child. Cureus 12(8), e9822 (2020)
Dignass, A., et al.: Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 6(10), 991–1030 (2012)
Rabbenou, W., Chang, S.: Medical treatment of pouchitis: a guide for the clinician. Therap Adv Gastroenterol 14, 17562848211023376 (2021)
Bamias, G., Arseneau, K.O., Cominelli, F.: Mouse models of inflammatory bowel disease for investigating mucosal immunity in the intestine. Curr Opin Gastroenterol 33(6), 411–416 (2017)
MacPherson, B.R., Pfeiffer, C.J.: Experimental production of diffuse colitis in rats. Digestion 17(2), 135–150 (1978)
Yamada, Y., et al.: A comparative analysis of two models of colitis in rats. Gastroenterology 102(5), 1524–1534 (1992)
Fiorucci, S., et al.: Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis. Immunity 17(6), 769–780 (2002)
Morris, G.P., et al.: Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96(3), 795–803 (1989)
Neurath, M.F., et al.: Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med 183(6), 2605–2616 (1996)
Cooper, H.S., et al.: Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69(2), 238–249 (1993)
Chassaing, B., et al.: Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104, 15 25 1-15 25 14 (2014)
Yamada, M., Ohkusa, T., Okayasu, I.: Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextran sulphate sodium. Gut 33(11), 1521–1527 (1992)
Elinav, E., et al.: NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145(5), 745–757 (2011)
Pizarro, T.T., et al.: SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis 17(12), 2566–2584 (2011)
Schulte, L., et al.: Intestinal organoids as a novel complementary model to dissect inflammatory bowel disease. Stem Cells Int 2019, 8010645 (2019)
Bouma, G., Kaushiva, A., Strober, W.: Experimental murine colitis is regulated by two genetic loci, including one on chromosome 11 that regulates IL-12 responses. Gastroenterology 123(2), 554–565 (2002)
Kiesler, P., Fuss, I.J., Strober, W.: Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 1(2), 154–170 (2015)
Garrett, W.S., et al.: Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131(1), 33–45 (2007)
Kuhn, R., et al.: Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75(2), 263–274 (1993)
Matsumoto, S., et al.: Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 43(1), 71–78 (1998)
Takeda, K., et al.: Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10(1), 39–49 (1999)
Zaph, C., et al.: Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446(7135), 552–556 (2007)
Li, X.G., et al.: Intestinal models for personalized medicine: from conventional models to microfluidic primary intestine-on-a-chip. Stem Cell Rev Rep 18(6), 2137–2151 (2022)
Pimenta, J., et al.: Organ-on-chip approaches for intestinal 3D in vitro modeling. Cell Mol Gastroenterol Hepatol 13(2), 351–367 (2022)
Dahlgren, D. and H. Lennernas, Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics, 2019. 11(8).
Jackson, E.L., Lu, H.: Three-dimensional models for studying development and disease: moving on from organisms to organs-on-a-chip and organoids. Integr Biol (Camb) 8(6), 672–683 (2016)
Perreault, N., Beaulieu, J.F.: Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res 245(1), 34–42 (1998)
Harper, K.D., Iozzo, R.V., Haddad, J.G.: Receptors for and bioresponses to 1,25-dihydroxyvitamin D in a human colon carcinoma cell line (HT-29). Metabolism 38(11), 1062–1069 (1989)
Yoo, J.H., Donowitz, M.: Intestinal enteroids/organoids: a novel platform for drug discovery in inflammatory bowel diseases. World J Gastroenterol 25(30), 4125–4147 (2019)
Finkbeiner, S.R., et al.: Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open 4(11), 1462–1472 (2015)
Spence, J.R., et al.: Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470(7332), 105–109 (2011)
Sato, T., et al.: Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244), 262–265 (2009)
Sato, T., et al.: Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5), 1762–1772 (2011)
Kraiczy, J., et al.: DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut 68(1), 49–61 (2019)
de Lau, W., et al.: Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts.” Mol Cell Biol 32(18), 3639–3647 (2012)
Suzuki, K., et al.: Single cell analysis of Crohn’s disease patient-derived small intestinal organoids reveals disease activity-dependent modification of stem cell properties. J Gastroenterol 53(9), 1035–1047 (2018)
Dotti, I., et al.: Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut 66(12), 2069–2079 (2017)
Howell, K.J., et al.: DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology 154(3), 585–598 (2018)
Barnicle, A., et al.: Inflammation-associated DNA methylation patterns in epithelium of ulcerative colitis. Epigenetics 12(8), 591–606 (2017)
Okamoto, R., Watanabe, M.: Investigating cell therapy for inflammatory bowel disease. Expert Opin Biol Ther 16(8), 1015–1023 (2016)
Borten, M.A., et al.: Automated brightfield morphometry of 3D organoid populations by OrganoSeg. Sci Rep 8(1), 5319 (2018)
Yu, F., W. Hunziker, and D. Choudhury, Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines (Basel), 2019. 10(3).
Noben, M., et al.: Human intestinal epithelium in a dish: current models for research into gastrointestinal pathophysiology. United Eur Gastroenterol J 5(8), 1073–1081 (2017)
Tong, Z., et al.: Towards a defined ECM and small molecule based monolayer culture system for the expansion of mouse and human intestinal stem cells. Biomaterials 154, 60–73 (2018)
Wu, L., et al.: Organoids/organs-on-a-chip: new frontiers of intestinal pathophysiological models. Lab Chip 23(5), 1192–1212 (2023)
Shin, W., Kim, H.J.: 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat Protoc 17(3), 910–939 (2022)
in The Impact of Food Bioactives on Health: in vitro and ex vivo models, K. Verhoeckx, et al., Editors. 2015: Cham (CH).
Jalili-Firoozinezhad, S., et al.: Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis 9(2), 223 (2018)
Cao, Y., et al.: The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol 37(12), 1359–1369 (2017)
Han, J., et al.: Imaging technologies for microfluidic biochips. BioChip J. 16(3), 255–269 (2022)
Gjorevski, N., et al.: Neutrophilic infiltration in organ-on-a-chip model of tissue inflammation. Lab Chip 20(18), 3365–3374 (2020)
Jang, M., Kim, H.N.: From single- to multi-organ-on-a-chip system for studying metabolic diseases. BioChip J. 17(2), 133–146 (2023)
Gijzen, L., et al.: An intestine-on-a-chip model of plug-and-play modularity to study inflammatory processes. SLAS Technol 25(6), 585–597 (2020)
Winkler, T.E., et al.: Low-cost microphysiological systems: feasibility study of a tape-based barrier-on-chip for small intestine modeling. Lab Chip 20(7), 1212–1226 (2020)
Elinav, E., et al.: The cancer microbiome. Nat Rev Cancer 19(7), 371–376 (2019)
Grassart, A., et al.: Bioengineered human organ-on-chip reveals intestinal microenvironment and mechanical forces impacting Shigella infection. Cell Host Microbe 26(3), 435–4444 (2019)
Yuan, L., et al.: Visualization of bacterial colonization and cellular layers in a gut-on-a-chip system using optical coherence tomography. Microsc Microanal 26(6), 1211–1219 (2020)
Kim, J., et al.: Microfabricated stretching devices for studying the effects of tensile stress on cells and tissues. BioChip J. 16(4), 366–375 (2022)
Maurer, M., et al.: A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 220, 119396 (2019)
Jeon, M.S., et al.: Contributions of the microbiome to intestinal inflammation in a gut-on-a-chip. Nano Converg 9(1), 8 (2022)
Kim, R.: Advanced organotypic in vitro model systems for host-microbial coculture. BioChip J. 17(2), 147–173 (2023)
Chang, J.T.: Pathophysiology of inflammatory bowel diseases. N Engl J Med 383(27), 2652–2664 (2020)
Karantanos, T., Gazouli, M.: Inflammatory bowel disease: recent advances on genetics and innate immunity. Ann Gastroenterol 24(3), 164–172 (2011)
Jostins, L., et al.: Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422), 119–124 (2012)
Huang, H., et al.: Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547(7662), 173–178 (2017)
Meddens, C.A., et al.: Non-coding DNA in IBD: from sequence variation in DNA regulatory elements to novel therapeutic potential. Gut 68(5), 928–941 (2019)
Antoni, L., et al.: Intestinal barrier in inflammatory bowel disease. World J Gastroenterol 20(5), 1165–1179 (2014)
Sunderhauf, A., et al.: Loss of mucosal p32/gC1qR/HABP1 triggers energy deficiency and impairs goblet cell differentiation in ulcerative colitis. Cell Mol Gastroenterol Hepatol 12(1), 229–250 (2021)
Lueschow, S.R., McElroy, S.J.: The paneth cell: the curator and defender of the immature small intestine. Front Immunol 11, 587 (2020)
Wu, G.D., et al.: Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052), 105–108 (2011)
Hviid, A., Svanstrom, H., Frisch, M.: Antibiotic use and inflammatory bowel diseases in childhood. Gut 60(1), 49–54 (2011)
Sekirov, I., et al.: Gut microbiota in health and disease. Physiol Rev 90(3), 859–904 (2010)
Borody, T.J., et al.: Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 37(1), 42–47 (2003)
Abreu, M.T., Fukata, M., Arditi, M.: TLR signaling in the gut in health and disease. J Immunol 174(8), 4453–4460 (2005)
Sanchez-Munoz, F., Dominguez-Lopez, A., Yamamoto-Furusho, J.K.: Role of cytokines in inflammatory bowel disease. World J Gastroenterol 14(27), 4280–4288 (2008)
Lu, Q., et al.: Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflamm Res 15, 1825–1844 (2022)
O’Connell, L., Winter, D.C., Aherne, C.M.: The role of organoids as a novel platform for modeling of inflammatory bowel disease. Front Pediatr 9, 624045 (2021)
Giorgio, C.D., et al., Modeling inflammatory bowel disease by intestinal organoids. Recent Adv Inflamm Allergy Drug Discov, 2022.
Iida, T., et al., Impact of Autophagy of Innate Immune Cells on Inflammatory Bowel Disease. Cells, 2018. 8(1).
Zuo, T., Ng, S.C.: The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol 9, 2247 (2018)
Yeshi, K., et al., Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med, 2020. 9(5).
Velasco, V., Shariati, S.A., Esfandyarpour, R.: Microtechnology-based methods for organoid models. Microsyst Nanoeng 6, 76 (2020)
Altay, G., et al.: In vitro self-organized mouse small intestinal epithelial monolayer protocol. Bio Protoc 10(3), e3514 (2020)
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MOE). (No. NRF-2021R1I1A3061265)
Funding
The National Research Foundation of Korea (Grant No. 2021R1I1A3061265), Sehoon Jeong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, J., Jeong, S. Inflammatory Bowel Disease: Pathophysiology, Treatment, and Disease Modeling. BioChip J 17, 403–430 (2023). https://doi.org/10.1007/s13206-023-00118-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-023-00118-y