Abstract
Metalloproteinases—such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinases (ADAMs)—are involved in various diseases of the nervous system but also contribute to nervous system development, synaptic plasticity and neuroregeneration upon injury. MMPs and ADAMs proteolytically cleave many substrates including extracellular matrix components but also signaling molecules and receptors. During neuroinfectious disease with associated neuroinflammation, MMPs and ADAMs regulate blood–brain barrier breakdown, bacterial invasion, neutrophil infiltration and cytokine signaling. Specific and broad-spectrum inhibitors for MMPs and ADAMs have experimentally been shown to decrease neuroinflammation and brain damage in diseases with excessive neuroinflammation as a common denominator, such as pneumococcal meningitis and multiple sclerosis, thereby improving the disease outcome. Timing of metalloproteinase inhibition appears to be critical to effectively target the cascade of pathophysiological processes leading to brain damage without inhibiting the neuroregenerative effects of metalloproteinases. As the critical role of metalloproteinases in neuronal repair mechanisms and regeneration was only lately recognized, the original idea of chronic MMP inhibition needs to be conceptually revised. Recently accumulated research urges for a second chance of metalloproteinase inhibitors, which—when correctly applied and dosed—harbor the potential to improve the outcome of different neuroinflammatory diseases.
Similar content being viewed by others
MMPs and ADAMs and their function in the CNS
Matrix metalloproteinases (MMPs) and A disintegrin and metalloproteinases (ADAMs) belong to the class of metzincin metalloproteinases containing a conserved Met residue at their active site and using a zinc ion during the enzymatic reaction. These metalloproteinases are involved in many neurological conditions but also contribute crucially to neurophysiological functions, such as synaptic plasticity and neuroregeneration via regulating stem cell biology and remyelination [1]. Until now, 24 different mammalian MMPs are described, while each type has a defined substrate specificity [2]. Collectively, MMPs can degrade all components of the extracellular matrix (ECM) [1, 3]. Apart from degrading ECM, MMPs are also able to cleave adhesion molecules, receptors and growth factors [4], indicating an involvement of MMPs in cell migration, signaling, differentiation, cell survival or apoptosis, angiogenesis and inflammation [1, 2, 5]. ADAMs also have the capacity to degrade and remodel components of the ECM, but their best characterized function is protein ectodomain shedding, thereby processing and releasing mature proteins (e.g., TNF-α) from membrane-anchored precursors [1, 6]. During pathological conditions of the central nervous system (CNS), metalloproteinases derive from infiltrating leukocytes (neutrophils, macrophages and lymphocytes) or brain-resident cells (microglia, astrocytes and neurons) [7].
Regulation of metalloproteinase activity
As metalloproteinases have the capacity for extensive tissue destruction, their activity is tightly regulated and controlled [1, 8]. The catalytic activity of MMPs is regulated on four different levels; namely gene expression, compartmentalization, pro-enzyme activation and enzyme inhibition [5]. Pro-MMPs are kept in a catalytically inactive state and can be activated by proteolytic cleavage of the pro-domain or by modification of the pro-domain’s cysteine thiol group [5, 9]. Of importance for inflammatory and infectious processes, reactive oxygen species (ROS) have the potential to activate MMPs via oxidation of the pro-domain’s thiol group [10,11,12]. MMP inactivation might be achieved be natural inhibitors, of which the tissue inhibitors of metalloproteinases (TIMPs) are the most prominent ones. By binding to the catalytic site of MMPs, the four TIMPs (TIMP-1, -2, -3 and -4) are able to terminate MMP activity [5, 13]. Many TIMPs target multiple metalloproteinases, but there are also some strict specificities such as TIMP-3, which is the only TIMP to inhibit ADAM17 [14, 15].
Effect of metalloproteinases on BBB integrity
The contribution of MMPs on blood–brain barrier (BBB) integrity was demonstrated for the first time in the early 1990s by inducing BBB breakdown after intracerebroventricular administration of MMP-2 and MMP-9, an effect that was prevented by simultaneous administration of TIMP-2 [16,17,18]. Subsequent findings of elevated MMP-9 levels after cerebral insults for the first time established a link between MMPs and inflammation in the CNS [18, 19]. Intracerebral injection of lipopolysaccharide (LPS)—mimicking bacterial brain infections—increased MMP-2, MMP-3 and TNF-α mRNA levels [20] and induced BBB disruption through the action of MMP-9 [21].
Blood–brain barrier opening is associated with a morphological redistribution of tight junction (TJ) and adherens junction (AJ) proteins from the membrane to the cytoplasm [22]. In different neurological pathologies, MMPs are reported to degrade TJ and basal lamina proteins, thereby inducing BBB leakage with subsequent neutrophil infiltration, brain edema and hemorrhage [22, 23].
The role of MMP-2 in BBB opening was intensively studied in cerebral ischemia models, where MMP-2 was found to play an important role in the initial BBB opening [24] with increased activation of MMP-2 being associated with the degradation of the TJ proteins claudin-5 and occludin [17, 25]. Both MMP-2 and MMP-9 (gelatinase A and B) are able to degrade the major components of the basal lamina (type IV collagen, laminin and fibronectin) surrounding the cerebral blood vessels, thus increasing BBB permeability [22]. In vitro and in vivo, MMP-9 is capable of degrade claudin-5, occludin and ZO-1, thereby participating in TJ degradation [25,26,27]. Experimental data propose that MMP-9—which plays a profound role in delayed BBB breakdown [28]—derives mostly from brain microvascular endothelial cells and infiltrating neutrophils [27, 29,30,31,32]. Infiltrating neutrophils contain stores of MMP-9—so-called tertiary granules (or gelatinase granules)—which are rapidly released upon entering the site of injury [1, 33, 34]. MMP-9 levels critically increase during bacterial meningitis and elevated MMP-9 levels are associated with BBB damage and neurological sequelae [32, 35]. In experimental ischemic stroke, increased neutrophil-derived MMP-9 is critically involved in a state of systemic inflammation with sustained degradation of TJ and basal lamina proteins leading to exacerbated brain injury [22, 27]. Apart from these two gelatinases, other MMPs influence BBB integrity. MMP-3 is able to degrade laminin, gelatin, E-cadherin, proteoglycans and fibronectin [36] and has been demonstrated to mediate BBB opening after LPS-induced neuroinflammation, thereby facilitating neutrophil infiltration [37]. Collagenases (MMP-1, -8 and -13) preferentially cleave helical collagens in the ECM [22] and are reported to be activated and/or upregulated in vessels with BBB impairment [38,39,40]. In bacterial meningitis, cerebrospinal fluid (CSF) levels of MMP-13 and MMP-8 are upregulated [41] and associated with BBB damage [35]. Repeated lumbar punctures in bacterial meningitis patients revealed that MMP-8 levels were regulated independently and did not correlate with CSF granulocyte cell counts, indicating that these MMPs might not only be secreted by infiltrating neutrophils but also from brain-resident cells [35].
Neural vascular barrier function is also regulated by ADAMs. ADAM12 and ADAM17 were shown to control neural vascular barrier function upon hypoxic stimuli by diminishing claudin-5 in brain microvascular endothelial cells, an effect that was prevented by specific inhibition of ADAM12 or ADAM17 [42]. Activated ADAM10 cleaves VE-cadherin and promotes leukocyte migration to inter-endothelial junctions [43, 44].
MMPs and ADAMs in progression of neuroinflammation
As the first described MMP was found to degrade collagen [45], the following contributions on MMPs mostly focused on their ability to degrade ECM components [5]. More recent data suggest, however, that MMPs have a much wider range of activity, including non-matrix substrates [4]. Instead of clustering MMPs together for their function in ECM degradation, Parks et al. suggest to categorize them rather according to their role in inflammation [5]. Table 1 and Fig. 1 summarize functions of selected metalloproteinases in pro- and anti-inflammatory processes.
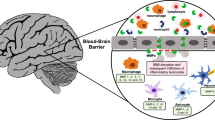
Pro-inflammatory roles of metalloproteinases during neuroinflammation. MMPs directly and indirectly contribute to chemotactic gradients in the bloodstream and neutrophil (orange cells) recruitment at site of infection. ADAMs and MMPs contribute to adherens junction protein (AJP) and tight junction protein (TJP) degradation. MMPs further show specific cleavage of basal lamina components. Upon destruction of the BBB, neutrophils enter the central nervous system and further generate chemotactic gradients with secreted MMP-9 being able to cleave and activate IL-8. Collagenases further contribute to BBB leakage by degradation of the extracellular matrix (ECM). ADAMs and MMPs directly contribute to neuroinflammation by activating pro-inflammatory cytokines like IL-1β and TNF-α from microglia/macrophage (green cell). This illustration only summarizes the most important roles of ADAMs and MMPs in induction of neuroinflammation, a more thorough picture is found in Table 1. For simplicity, the BBB is depicted without pericytes and astrocytes. Illustration design inspired by Khokha et al. [15]
MMPs in neutrophil migration and cytokine signaling
MMPs in chemokine signaling
To establish an effective immune reaction against invading pathogens, neutrophils must migrate efficiently along chemotactic gradients and extravasate from the blood vessels towards the site of infection. Metalloproteinases have been demonstrated to be involved in these processes not only by degrading ECM and promoting effector cell extravasation but also by regulation of chemotactic gradients [15]. This was first demonstrated with MMP-9 shown to process an amino-terminal fragment of IL-8, thereby increasing its chemoattractant properties to more efficiently recruit neutrophils [46]. On the other hand, MMP-2 was shown to inactivate monocyte chemotactic protein 3 (MCP3, also known as chemokine (C–C motif) ligand 7, CCL7) by removing an amino-terminal tetrapeptide, converting it to an antagonist of its chemokine receptors [47]. Conceptually, these findings showed that MMPs can not only act as effectors but also regulators of inflammatory responses [47]. Subsequently, CCL7 has been also shown to be a specific substrate of MMP-1, -3, -13 and -14 but not MMP-8 and -9 [48]. The closely related chemokines CCL2, CCL8 and CCL13 (MCP-1, -2 and -4) are proteolytically cleaved by MMP-1 and -3 (but not MMP-2 and -14) with the truncated products of CCL8 and CCL13 being potent antagonists of the their respective chemokine receptors [48]. Apart from influencing the activity of C–C motif chemokines, MMPs also contribute to CXC-chemokine function. Stromal cell-derived factor 1 alpha (SDF-1α, also known as CXCL12) is processed and inactivated by MMP-1, -2, -3, -9, -13 and -14 but not by MMP-7 and -8 [49]. The truncated form of CXCL12 after MMP-2 processing demonstrates highly neurotoxic properties [50]. An elegant study with single and double knockout mice for MMP-2 and MMP-9 revealed that these MMPs work synergistically in the initial step of neutrophil recruitment to injury sites by increasing the potency of CXC-chemokine ligand 5 (CXCL5) [51]. Activation of murine LPS-induced CXC chemokine (LIX, similar to the human neutrophil-recruiting chemokine CXCL5 and CXCL8/IL-8) is dependent on MMP-8, with cleaved LIX promoting enhanced chemotaxis [52]. Neutrophil infiltration at LPS-stimulated sites is clearly diminished in mmp8−/− mice [52]. The same study showed that MMP-8 itself is not required for the extravasation and migration of neutrophils, but plays a very crucial role in orchestrating the initial inflammatory response upon LPS stimulation, thereby indicating that chemoattractants rather than collagen are MMP-8’s primary substrates [52]. Nevertheless, collagens processed by MMP-8 can also act as chemotactic peptides during neutrophil chemotaxis [53]. Both MMP-8 and -9 are reported to cleave CXCL5 and CXCL6, but no change in biological activity of CXCL6 was demonstrated [54]. MMP-9, in contrast, potently inactivates CXCL5 by multiple cleavages, suggesting a regulatory role of MMP-9 in early activation with subsequent inactivation of CXCL5 [54]. In addition to direct effects of MMPs on chemokines, MMPs might also regulate the expression of chemokine receptors, thereby providing an additional level by which these proteinases control leukocyte migration [5, 55].
Besides directly processing chemokines and altering their biological function, MMPs can also indirectly influence the availability and activity of chemokines by cleaving accessory macromolecules that bind them [56]. Thereby, MMPs exhibit an additional way of regulating leukocyte migration [5]. MMP-7 is involved in syndecan-1 cleavage, which in turn releases CXCL1 (also known as KC). Thereby, MMP-7 indirectly generates a chemotactic gradient that directs neutrophil migration to sites of injury [56]. Consequently, neutrophil infiltration is massively reduced in mmp7−/− mice at sites of injury because of impaired transepithelial migration [56]. Other MMPs also regulate chemotactic gradients, but their role is less well understood. MMP-2 seems to be critical for establishing a CCL11 (eotaxin) chemotactic gradient during leukocyte recruitment, as allergen-induced asthmatic mmp2−/− mice show leukocyte accumulation in lung parenchyma and decreased CCL11 levels [57]. Notably, this indirect pro-inflammatory effect of MMP-2 is very distinct from the above discussed direct anti-inflammatory function via chemokine cleavage and inactivation [47, 49]. In addition to MMP-2, MMP-9 contributes to the generation of the chemotactic gradient in allergen-induced asthmatic mice, where mmp9−/− mice showed diminished CCL11, CCL7 and CCL17 chemoattractant levels [58]. In summary, these studies demonstrate that MMPs are important regulators of inflammation by controlling chemokine activity and chemotactic gradients, thereby manipulating leukocyte migration.
MMPs in cytokine signaling
Apart from generating chemotactic gradients and recruiting leukocytes to sites of infection, MMPs also have the capacity to directly activate and regulate cytokine signaling. The pro-inflammatory cytokine IL-1β needs proteolytic activation by the IL-1β-converting enzyme (ICE, more recently re-named to caspase-1) [59, 60]. After discovery of caspase-1-independent activation of IL-1β [61], MMP-2, -3 and -9 were described to process human IL-1β precursor into biologically active forms [62]. In addition, MMP-3—to a lesser extent MMP-9, but not collagenases—was found to degrade active IL-1β after longer incubation periods, indicating regulatory roles of MMPs in both IL-1β activation and inactivation [62]. Under physiological conditions, conversion of the membrane-bound pro-TNF-α to its active and soluble form is attributed to TNF-α-converting enzyme (TACE, now known as ADAM17, also see next section) [63, 64]. Even if ADAM17 is the most specific and best convertor of pro-TNF-α, MMP-7—and to lesser but not physiologically relevant extent MMP-1 and -9—harbors the capacity to cleave and activate pro-TNF-α and seems to act as another physiological TNF-α convertor [65]. In contrast to the activation of these pro-inflammatory cytokines, MMPs (MMP-3, -9 and -14) are also able to activate transforming growth factor-β1 (TGF-β1) in vitro [66,67,68], which restrains mononuclear inflammation [5, 69, 70], thereby again indicating an overall regulatory effect of MMPs in inflammation.
ADAMs in infection and inflammatory responses
TNF-α-converting enzyme (TACE; ADAM17) was originally thought to be a MMP, as it was effectively inhibited by synthetic metalloproteinase inhibitors [71], but later found to belong the ADAM family and identified as ADAM17 [63, 64]. ADAM17 shows very distinct specificity for shedding membrane-bound pro-TNF-α, thereby releasing biologically active TNF-α [65], with both pro-inflammatory and pro-apoptotic functions [15]. In ADAM17-deficient cells, TNF-α release is reduced by 90%, indicating that ADAM17 is the principal TNF-α converting enzyme [5, 64]. Soluble TNF-α signals via binding of TNF receptor 1 (TNFR1), whereas membrane-bound TNF-α acts preferentially through binding of TNFR2 [72]. The regulation of TNF signaling by ADAM17 is even more complex, since ADAM17 also sheds TNFR1 and TNFR2 [73, 74]. These act as decoy receptors by sequestering soluble TNF-α away from their receptors [15]. ADAM17 deficiency in myeloid cells protected mice from endotoxin-induced septic shock by preventing increased serum levels of TNF-α [75]. As ADAM17-dependent TNF-α conversion is selectively inhibited by TIMP3 [14], Timp3−/− mice show enhanced TNF-α signaling with elevated IL-6 serum levels and increased mortality after LPS-induced sepsis [76]. ADAM17—but also ADAM10—have been described to be involved in IL-6 trans-signaling by shedding IL-6 receptor (IL-6R), which in turn complexes with IL-6 and interacts with gp130-containing membrane receptors that are ubiquitously expressed on many cell types [77, 78].
Most leukocytes express L-selectin on their surface, which is involved in their rolling on inflamed vascular endothelium, followed by firm adhesion and transmigration [79]. L-selectin is rapidly cleaved near the leukocyte cell surface by ADAM17 [79], during their transmigration [15]. ADAM17-null neutrophils show slower rolling, better adhesion and faster recruitment to site of inflammation [80] with impaired L-selectin shedding being responsible for early neutrophil recruitment [80, 81].
In mucosal barriers, ADAM17 has been shown to be important for EGFR activation, with ADAM17 or EGFR-deficient mice showing similar features [74]. Subsequently, ADAM17 was shown to be the major convertase of the EGFR ligands epiregulin, TGF-α, amphiregulin and heparin-binding EGF-like growth factors, whereas ADAM10 emerged as the major sheddase of EGF and betacellulin [82]. In intestinal inflammation, ADAM17-dependent EGFR ligand shedding is necessary for the production of antimicrobial peptides by epithelial cells and later regeneration [15, 83]. ADAM17 has additionally been associated with shedding of L-selectin from activated T cells, thereby regulating the recruitment of adaptive immune cells [74].
ADAMs are also able to manipulate immune signaling via ectodomain shedding of the Notch receptor, which is required for its activation [84, 85]. Notch activation by ADAM17 was found to regulate atopic barrier function and suppress epithelial cytokine synthesis [86]. In addition, ADAM17 and ADAMTS12 were shown to be involved in neutrophil apoptosis during resolution of acute inflammation [87, 88].
MMPs and microglia
Microglia are brain-resident immune cells that are involved in important roles of the healthy, infected and injured brain, including post-natal neurodevelopment, neural plasticity and phagocytosis [89, 90]. Upon stimulation, microglia can be polarized into different microglial subsets [91]. The polarization states can be roughly divided into classically activated (M1) microglia that adapt a pro-inflammatory phenotype by secreting TNF-α, IL-1β, IL-6 and IFNγ [92], and alternatively activated (M2) cells, which produce cytokines involved in inflammation termination, restoring homeostasis and promoting tissue repair [91]. MMPs are expressed and produced by microglia at site of infection and inflammation [93, 94]. In macrophages, the two different subsets—M1 and M2—express different MMPs [95]. MMP-9—which depicts pro-inflammatory roles in BBB opening and cytokine activation—is secreted by M2 microglia and involved as a remodeling factor during repair [96, 97]. Kohkha et al. further suggest that MMPs—apart from being differentially expressed in M1 and M2 subsets—might also contribute to phenotype polarization by regulating cytokine and growth factor availability [15]. As microglia polarization towards the M2 phenotype gains interest as therapeutic strategy for different neurological disorders, understanding the critical role of metalloproteinases during this process needs further investigations.
Collectively, these findings demonstrate the crucial role of metalloproteinases in initiation of neuroinflammation—including BBB breakdown, chemokine activation with neutrophil recruitment, and pro-inflammatory cytokine production—but also in inflammation termination and subsequent repair via chemokine and cytokine inactivation and involvement in angiogenesis, neurogenesis and gliosis after damage [98,99,100,101]. Therefore, inhibition of metalloproteinase during acute inflammatory processes can lead to beneficial outcomes, whereas inhibition during repair processes might be detrimental [102, 103].
MMPs and ADAMs in infectious disease of the CNS
The brain is protected from infectious agents by specialized barriers including the skull, the meninges and the restrictive BBB, making the brain a microbiologically sterile site under physiologic conditions [104]. Subsequently, infectious disease of the CNS—such as bacterial meningitis—occur at comparatively low levels but might have detrimental consequences [105]. As described above, metalloproteinases regulate barrier functions of the BBB but also of mucosal epithelium and play multiple roles in the initiation and regulation of inflammation, thereby being essentially involved in CNS infections.
Metalloproteinases in bacterial meningitis
Pathophysiology of brain injury during bacterial meningitis
To establish a CNS infection, the bacterial pathogens have to successfully colonize the host before they gain access to the subarachnoid space or the brain parenchyma. The most common causative agents of bacterial meningitis—Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae type b [106]—colonize the human nasopharynx and are transmitted via the respiratory route [105]. Most cases of bacterial meningitis originate from bacteremia, where the nasopharyngal pathogen gains access to the blood stream and subsequently crosses the BBB or blood–CSF barrier (BCSFB). Other causative pathogens of bacterial meningitis—which cause meningitis mostly in neonates, elderly and immunocompromized patients (group B Streptococcus (GBS), Escherichia coli, Listeria monocytogenes)—colonize the intestinal mucosa from where they access the circulation and invade the brain [105]. Bacterial meningitis might also arise from focal infection in the vicinity of the brain (i.e., otitis media, sinusitis, dental abscess), where local spread rather than bacteremia underlies bacterial brain invasion [105]. Finally, breaches of the anatomical brain barriers (trauma or surgery) may also lead to bacterial meningitis.
Among bacterial meningitis, pneumococcal meningitis (PM) is especially detrimental as it causes high mortality and leads to long-lasting neurofunctional deficits [107, 108]. Neurofunctional sequelae after PM include hearing loss, epilepsy, cerebral palsy, as well as behavioral and cognitive deficits [107,108,109]. Brain damage during PM is characterized by cortical necrosis and apoptosis of dentate gyrus granular cell progenitors in the hippocampus, being in part responsible for behavioral and cognitive deficits [110,111,112,113,114]. In experimental models, cortical necrosis is found as early as 18 h after infection and might arise as a consequence of focal and global ischemia [107, 115]. Neural cell death is caused by multiple factors including bacterial toxins and an excessive inflammatory reaction from the host [105, 116,117,118]. Inflammatory mediators (cytokines, chemokines, reactive oxygen and nitrogen species, MMPs) released from recruited neutrophils, endothelial cells, leptomeningeal macrophages and brain-resident microglia and astrocytes contribute to pathogen eradication but also act as neurotoxins and induce neuronal damage [107, 116, 119, 120]. Moreover, PM induces damage to hair cells and spiral ganglion neurons in the inner ear [121,122,123] provoking sensorineural hearing impairments in up to 30% of survivors [108, 124, 125]. In an infant rat PM model, increased CSF levels of TNF-α during acute infection were positively correlated with increased hearing loss in surviving animals [122].
The pathogenesis and involvement of inflammatory processes in inducing brain damage during meningitis have been intensively studied in different animal models. Upon bacterial invasion into the CNS, a wide range of cytokine is produced and secreted into the CSF [126]. In experimental pneumococcal meningitis, increased CSF levels of TNF-α and MMP-9 are already detected at 4 h after infection and peaked at 12 h after infection [127]. Since levels of TNF-α, IL-1β, IL-6 and IL-8 in the CSF increase before evidence of neutrophil recruitment, brain-resident cells are considered to contribute significantly to cytokine production [126]. As a result of bacterial proliferation, an excessive inflammatory reaction takes place in the CSF with BBB breakdown causing brain edema via leakage of plasma into the CNS, increased intracranial pressure, hydrocephalus and cerebral ischemia [116]. In patients with bacterial meningitis, cerebrovascular complications are frequently observed [124] with vasculitis being the cause for cerebral infarction, hemorrhages and subsequent cortical damage during meningitis [18, 116, 124].
Metalloproteinases in the pathogenesis of bacterial meningitis
Data from clinical and experimental studies have shed light onto the crucial role of metalloproteinases during pathogenesis of bacterial meningitis and other neuro-infectious diseases. Metalloproteinases—expressed or activated in response to the invading pathogen—contribute to acute neuroinflammatory reaction and damage upon bacterial invasion but might also be involved later during the disease in the resolution of inflammation and repair mechanisms [41, 127,128,129]. During bacterial meningitis, the expression and activation of metalloproteinases and their inhibitors are altered compared to physiologic conditions. During experimental PM in infant rats, MMP-8 and MMP-9 are transcriptionally upregulated in CSF cells, whereas the expression of MMP-2 and MMP-7 remains unaffected. In brain tissue, expression of MMP-3, -8, -9, -12, -13 and -14 was significantly upregulated (100- to 1000-fold), while expression levels of MMP-2 and MMP-7 remained unchanged [127]. The upregulation of MMP-8 (mostly released from infiltrating neutrophils) is a specific hallmark of bacterial meningitis, which is not found in other neuroinflammatory disease [130]. MMP-8 induces increased barrier permeability via proteolytic cleavage of the tight junction protein occludin in meningococcal neuroinfection [131]. Other experimental data of meningitis caused by heat-inactivated N. meningitidis showed a significant upregulation of MMP-9 mRNA expression with stable MMP-2 and MMP-7 expression [41]. On the protein level, MMP-9 in the CSF correlated with TNF-α levels, with a concentration peak for both at 12 h after intracisternal infection with S. pneumoniae [127]. MMP-9 in the CSF was detected as early as 15 min after intracisternal infection, indicating its early release from brain-resident cells in this experimental model. Further recruitment and infiltration of neutrophils contribute to the peak MMP-9 levels at 12 h after infection [127]. Gelatinase activity (MMP-2 and/or MMP-9) has been associated with the occurrence of cortical necrotic lesions in experimental PM [23, 132]. During the initiation of neuroinflammation, ADAM17 plays a crucial role in releasing TNF-α, which in turn acts as a stimulus to induce MMP upregulation via a positive feedback loop [127, 133]. The fact that MMP-7—being the second most potent TNF-α activator apart from ADAM17 [65]—remains unchanged in bacterial meningitis [127] emphasizes the importance of ADAM17 during this early neuroinflammatory process. In addition to increased TNF-α and MMP-9 levels, TIMP-1 expression is also increased in the CSF of infant rats with PM, however, with a short delay [23]. TIMPs are suggested to regulate protein degradation and cytokine shedding during PM, thereby controlling metalloproteinase-induced neuroinflammation. In experimental PM, the upregulation of MMP-9, however, exceeds the compensatory effect of TIMP-1 during the acute phase. This imbalance between MMPs and TIMPs during bacterial meningitis has, therefore, been implicated as a key event during pathophysiology of the disease [23]. In an experimental model of cerebral ischemia, MMP-9-deficient mice showed BBB preservation and reduced levels of tight junction protein degradation with subsequent better outcome [134]. MMP-9 deficiency during experimental PM, however, was shown to be associated with impaired bacterial clearance from blood and spleen, without showing an impact on clinical course of the disease, leukocyte infiltration of the subarachnoid space or bacterial titers in the brain [135]. Non-infectious experimental models of brain damage have shown a direct effect of MMP-9 on laminin degradation and associated hippocampal apoptosis [136, 137].
Matrix metalloproteinase expression profiles in patients with bacterial meningitis are consistent with the data generated in experimental settings. In patients with bacterial meningitis, CSF levels of MMP-1, -3, -7, -8, -9 and -10 have been found to be elevated, whereas CSF levels of MMP-2 remain unaffected [35, 138,139,140,141,142,143]. Similar to animal models, TIMP-1 levels have also been found to be upregulated, whereas TIMP-4 was significantly downregulated compared to control patients [142]. One week after infection, TIMP-1 continues to be highly expressed, whereas MMP-9 is reduced compared to the acute phase of infection [144]. In pediatric PM, MMP-9 levels were found to correlate with CSF cell counts, with high CSF MMP-9 levels being a risk factor for fatal outcome or development of neurologic sequelae [35, 141, 144]. The causative pathogen determines the inflammatory response in patients with bacterial meningitis. Pneumococcal meningitis is associated with significantly increased mortality and elevated levels of IFN-γ, MCP-1 and MMP-9 compared to meningitis caused by N. meningitidis or H. influenzae [145].
Tuberculous meningitis (TBM), which arises rarely as an extrapulmonary form of tuberculosis, is associated with neurofunctional complications [146]. In patients with TBM, levels of MMP-2 and MMP-9 were found to be elevated, and high levels of MMP-9 were associated with late neurofunctional deficits [147, 148]. Adjuvant dexamethasone during TBM was shown to reduce MMP-9 levels early in treatment and might represent one way by which dexamethasone reduces mortality in TBM [149, 150]. Despite the beneficial effect of dexamethasone in terms of mortality, it could not successfully prevent severe disabilities [150].
MMPs and ADAMs in viral neuro-infectious diseases
The involvement of metalloproteinase in BBB integrity during viral neuroinfections has been intensively reviewed elsewhere [151]. Clinical data from patients with viral meningitis report elevated CSF levels of MMP-9 and TIMP-1 compared to control patients, with MMP-9 levels correlating to neutrophil cell number in CSF [152]. Involvement of metalloproteinases during viral neuroinfection has mostly been analyzed in experimental studies, though.
Japanese encephalitis virus (JEV) is an arthropod-borne virus and a major cause of acute encephalopathy in children [153]. In vitro, MMP-9 expression after JEV infection is mediated via NF-κB activation and the generation of ROS [153]. The expression of MMP-2, -7 and -9, as well as TIMP-1 and -3 is upregulated in mice infected with JEV and their overexpression is associated with disease severity [154].
In experimental herpes-simplex virus encephalitis (HSE), MMP-9 is upregulated and insufficiently counterbalanced by TIMP-1 resulting in a loss of collagen type IV, indicating MMP-9’s role during the pathogenesis of HSE [155].
In vitro, West Nile virus (WNV)-infected human endothelial cells show significant upregulation of multiple MMPs with subsequent loss of tight junction proteins, an effect successfully prevented by the MMP inhibitor GM6001 [156]. MMP-9 levels are also found to be elevated in WNV-infected mice as well as in patients, suggesting that MMP-9 plays a role in mediating WNV entry into the CNS [157].
During HIV infection, a CCL2-dependent mechanism was postulated to be involved in infiltration of HIV-infected leukocytes into the CNS causing neuropathology. In vitro, this mechanism seems to rely on MMP-2 and -9 upregulation with an associated reduction of tight junction proteins [158]. In experimental HIV infection, the envelope protein gp120 increased MMP-2 and -9 expression with subsequent laminin and claudin-5 reduction and causing increased BBB permeability [159]. In patients with HIV-associated dementia, CSF levels of MMP-2, -7 and 9 are increased and might reflect a link to symptomatic neurological disease [160, 161]. Increased MMP activity with its detrimental effects on BBB integrity might contribute to transendothelial migration of HIV-infected cells into the CNS and development of HIV-associated neurologic damage [161].
Mouse adenovirus type-1 (MAV-1) infection increases levels of MMP-2 and -9 in brains [162]. Additional ex vivo data revealed that MMP-2 and -9 are produced by astrocytes and microglia in response to mouse encephalitic adenovirus-1, which might contribute to BBB disruption and encephalitis [163]. MAV-1 may also induce BBB breakdown by reducing surface expression of TJ proteins occludin and claudin-5 [164]. Murine coronavirus-induced viral encephalitis induces expression of MMP-3 and -12 plus TIMP-1, with MMP-3 expression exclusively localized in astrocytes, whereas TIMP-1 originates from infiltrating cells [165]. Increased MMP and TIMP levels are also associated with increased viral replication during neurotropic mouse hepatitis infection [166].
MMP and ADAM inhibitors as potential treatment options in bacterial meningitis
Since MMPs and ADAMs are central regulators and mediators during neuroinflammation and development of neuronal damage in bacterial meningitis, inhibition of theses enzymes during acute infection has been postulated as a promising therapeutic intervention to improve disease outcome. Over the last three decades, many different inhibitors were tested in rodent models of bacterial meningitis (Table 2).
Neuroinflammation and BBB breakdown
Matrix metalloproteinases and ADAMs function as ECM degrading enzymes and sheddases, thereby controlling BBB breakdown and production of inflammatory cytokines [15]. In experimental bacterial meningitis, MMP and ADAM inhibitors significantly reduce CSF levels of MMPs and pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-10) [128, 132, 167,168,169]. Notably, CSF levels of TNF-α are also significantly reduced by MMP inhibitors (MMPIs) without specificity for ADAM17 (i.e., Trocade) [167], indicating that MMPIs are able to indirectly reduce pro-inflammatory cytokine production apart from direct ADAM17 inhibition. MMPIs partially preserved BBB integrity by preventing ECM protein degradation [132, 167]. In a PM mouse model, TNF484 reduced neuroinflammation, thereby improving survival in animals without antibiotic therapy [170]. Notably, there are remarkable differences in different mouse and rat strains in terms of BBB breakdown prevention by metalloproteinase inhibitors after LPS-induced neuroinflammation [171].
Cortical necrosis
As a consequence of bacterial meningitis and its associated inflammatory reaction, cerebrovascular complications are frequently observed in patients [124]. Focal ischemia caused by vasculitis results in cortical necrosis during meningitis [116, 124]. Breakdown of the BBB during the excessive neuroinflammatory reaction is a crucial step in the development of cerebral ischemia [116]. Since metalloproteinases are central regulators of BBB breakdown during bacterial meningitis (discussed above), their inhibition during the acute disease is a valuable therapeutic strategy to reduce cortical necrosis. Pre-treatment or treatment early in the course of disease with MMPIs is associated with reduced cortical necrosis in pneumococcal meningitis [128, 132, 167, 168, 172, 173] and with lower rates of intracerebral hemorrhage in meningococcal meningitis [169]. This neuroprotective effect is, however, reduced when the application of the inhibitors is delayed until the time of antibiotic therapy at the first appearance of disease symptoms [127, 174]. The early inhibition of MMPs during bacterial meningitis might be responsible for an overall reduction of the neuroinflammatory reaction with reduced BBB breakdown and cytokine production, thereby limiting the pathophysiological consequences of the disease [116].
Hippocampal apoptosis
Apoptosis of immature neurons in the dentate gyrus of the hippocampus during bacterial meningitis is caused by bacterial toxins, but also by an excessive inflammatory reaction from the host [105, 116,117,118]. Several metalloproteinase inhibitors that inhibit ADAM17 (i.e., BB1001, Ro 32-7315) were found to prevent hippocampal apoptosis [127, 167]. However, other ADAM17 inhibitors were unable to prevent neural cell death in the hippocampus [132]. On the other hand, Trocade—without having a specific ADAM17-inhibitory profile—successfully prevented hippocampal apoptosis [167, 174]. As MMP-9 is directly involved in hippocampal cell death via the degradation of laminin [136, 137], inhibition of MMP-9—rather than ADAM17—might explain this neuroprotective effect of Trocade during bacterial meningitis. The protective effect on hippocampal neurons is lower—but still present—when BB1001 or Trocade therapy is initiated together with antibiotics at time of symptom onset [127, 167, 174].
Neurofunctional outcome
Apoptosis of dentate gyrus granular cell progenitors found in the hippocampus of humans [114] and animal models with bacterial meningitis [175, 176] correlates with behavioural and learning deficits [110,111,112,113], with increased hippocampal apoptosis being associated with impaired learning and memory performance in rat PM [111, 113]. Protection from hippocampal apoptosis by BB1001 improved learning performance in rats with PM [127]. Similarly, in PM-infected rats treated with Trocade plus daptomycin, the observed reduction in hippocampal apoptosis was associated with a significant improvement of learning and memory function [174]. GM6001 therapy significantly protected hippocampal neurons and prevented PM-induced learning and memory impairments in neonatal rats [177]. Notably, all three therapies also significantly protected from cortical necrosis [127, 174, 177], which might also positively influence learning and memory performance after PM. Correspondingly, in adult rats, MMP-2 and -9 inhibition further prevented cognitive impairment after PM possibly due to the observed successful preservation of BBB integrity during acute infection [178].
Bacterial meningitis-induced hearing loss
Bacterial meningitis induces sensorineural hearing loss by damaging hair cells and spiral ganglion neurons in the inner ear [122, 123]. In PM, CSF levels of TNF-α during acute infection are positively correlated with increased hearing threshold after infection [122]. Treatment with doxycycline or Trocade plus daptomycin significantly reduces CSF levels of TNF-α and other pro-inflammatory cytokines and significantly improves hearing thresholds after PM [173, 174]. Doxycycline—with its broad spectrum MMP inhibition profile—preserves spiral ganglion neurons from PM-induced cell death [173]. The overall reduction of neuroinflammation in both therapies might be attributable to a reduced concentration of ototoxic compounds in the cochlear duct, thereby preventing ototoxic damage in spiral ganglion neurons and hair cells of the cochlea [174].
Detrimental effects after metalloproteinase inhibition
Two metalloproteinase inhibitors—Ro 32-7315 and high-dose TNF484, both with high specificity for ADAM17—were reported to increase mortality during acute bacterial meningitis when administrated early after infection [132, 167]. In an experimental model of PM with TNF-α-deficient mice, mortality was significantly increased despite treatment with antibiotics, indicating that TNF-α may play an essential role during infection and inflammation, being necessary for host defense in the early stages of infection [179]. In PM, TNF-α inhibition by very potent ADAM17 inhibitors might, therefore, be rather detrimental than beneficial despite being neuroprotective in survivors, especially when applied at a time when TNF-α is a major contributor of the initial host response.
Matrix metalloproteinases in multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease of the CNS, leading to progressive deterioration of motor, sensory, vegetative and cognitive functions [180, 181]. The pathogenesis of MS encompasses both features of acute and subacute inflammation, and of chronic neurodegeneration, mainly as a result of the former. Because of these similarities with neuroinfectious disease with subsequent neuroinflammation and brain damage, MS is also covered in the present review.
There are more than 2.3 million patients worldwide, making MS the most important neurological diseases of young adulthood [180]. The disease affects predominantly Caucasians with highest prevalence in populations of Northern European origin. The cause of MS remains unknown, while there is strong evidence for an interplay of genetic and environmental factors (smoking, lack of sun exposure/vitamin D deficiency, Epstein–Barr virus (EBV) infection) responsible for susceptibility and course of disease [180, 181]. The classical understanding of MS pathogenesis is based on three essential paradigms. (1) Focal demyelination in the white matter is the predominant pathogenetic feature. (2) MS occurs in subtypes that are pathogenetically distinct from each other: primary progressive MS (PPMS) with development of disability from onset of disease; relapsing–remitting MS (RRMS) with alternation between acute deterioration and clinical stable phases; and secondary progressive MS (SPMS) with continuous disability worsening between or without intermittent relapse in patients earlier diagnosed with RRMS. (3) MS is a T cell dependent disease.
However, in recent years an increasing body of evidence has led to a modification and extension of these paradigms. Demyelination is not restricted to white matter, but extends steadily into the grey matter in the course of disease [182]. Neuronal loss as the cellular substrate of disability worsening occurs in the course of demyelination, but to an even larger extent outside and independent of focal demyelination. Additionally, it seems that a relevant fraction of patients have a form of MS where no white matter demyelination occurs [183]. Further, the phenotypic characterization of MS subtypes is not based on distinct molecular mechanisms. According to the current understanding, there is a continuum of several pathogenetic pathways, which may predominate at certain stages and disease duration [180]. Lastly, the success of B-cell targeting therapies has revolutionized our understanding on the autoimmune response in MS: B cells seem to orchestrate and drive the disease directly and indirectly via T cells and other cells in acute and progressive phases [184].
The important role of MMPs as effector molecules of inflammatory tissue damage has been recognized in the 1990s of the last century. The progress of our understanding of MS pathogenesis has rather increased their importance and added to the complexity of their regulation in the pathogenesis of disease.
Focal opening of the BBB is one of the initial events in the course of acute exacerbation of MS, followed by invasion of blood-derived leucocytes into the perivascular brain parenchyma, eventually leading to the formation of focal lesions that manifest clinically as attacks. Leukocytes, as well as cells that form the BBB (endothelial cells, astrocytes), produce MMPs and TIMPs, and BBB leakage is a result of MMP upregulation that is not any more compensated by TIMP activity [185]. Basal membrane type IV collagen as the primary barrier structure at the BBB is a main substrate for gelatinases (MMP-2 and -9), which makes them specific effector molecules of leukocyte extravasation into the brain parenchyma. In MS lesions, upregulation of MMP-1, -2, -3, -7 and -9 was demonstrated by immunohistochemistry [186,187,188] and on the transcriptional level [189], and correlated with inflammatory activity in lesions.
In the experimental autoimmune encephalitis (EAE) model of MS, the upregulation of MMP-7 and -9 correlated with the course of disease severity [190]. In contrast, knockout mice deficient for MMP-9 were less susceptible to EAE induction [191]. In the delayed-type hypersensitivity (DTH) rat model, protein and transcriptional expression of several MMPs was increased. Moreover, microinjection of MMP-7, -8 and -9 into brain parenchyma led to demyelination and axonal damage, the latter in part independent of the former. Axonal damage continued even when the integrity of the BBB was re-established [192, 193]. The fact that MMPs may cause direct neuronal damage is very important for the concept of clinical progression of MS. Their increased expression also in chronic phases of lesions development [189] may contribute to chronic smouldering neuronal loss, and eventually lead to progressive deterioration of neurological functions. On the other hand, CSF analysis in patients with ‘active progressive MS’ (having either a relapse the previous year or presence of lesions with gadolinium enhancement at baseline MRI) has higher levels of MMP-9 and CXCL13 (a B-cell activation marker) than inactive progressive MS patients. This coincides with higher levels of neurofilament light chain as a measure of ongoing axonal damage, and of MBP as indicator of demyelination [194].
Several groups have demonstrated an increase of MMP-9 in CSF [195, 196] and in blood of MS patients [185, 197]. The levels correlated with disease activity (higher in RRMS/SPMS vs PPMS [195], or MRI activity [185, 197]. In a longitudinal study of SPMS patients, the increase of MMP-9/TIMP-1 ratio preceded the occurrence of new gadolinium-enhancing lesions [198]. This study showed also higher median levels of TIMP-1. MMP-9 trended lower in patients receiving IFNβ compared to placebo recipients. Similarly, steroids treatment used as interventional therapy during acute exacerbation of MS led to a decrease of MMP-9 in CSF with the most prominent effect in patients with gadolinium-enhancing lesions, further supporting its functional role in BBB breakdown [199]. Moreover, quantitative PCR analysis revealed that IFNβ therapy downregulated the expression of MMP-2 and -9 in blood leukocytes. Patients that developed neutralizing antibodies against IFNβ showed an increase of these MMPs in circulation, supporting the concept that these antibodies reduce the therapeutic efficacy IFNβ therapy [200].
Most work on MMPs in MS is restricted to MMP-9 and -2. This is mainly due to the fact that these members of the MMP family could be quantified easily and with highest sensitivity by zymography, while for all other MMPs ELISAs were needed and became only available over the years. Bar-Or et al. performed a comprehensive analysis of transcriptional expression of 23 MMPs by quantitative PCR in monocytes as well as in B and T cells from healthy controls and MS patients [201]. Each of these cell types had their specific pattern of enzymes across the involved members of the MMP family. A specific increase of MMP-2 and -14, and of TIMP-2 in monocytes of MS patients was observed. Kouwenhoven et al. found that mRNA levels of MMP-1, -3, -7, -9 and of TIMP-1 in monocytes were elevated in MS, while those of MMP-14 did not differ, as compared to controls [202]. These studies underline that molecular mechanisms of tissue degradation are not restricted to only MMP-9, but result from an interplay of many MMPs from many cellular sources. Nevertheless, while results from different investigators show a high degree of congruency, there are discrepancies that may reflect the heterogeneity of characteristics of MS patients investigated.
Initially, MMPs were considered ‘good for physiologic growth, but bad in MS’. As shown in Table 1, this overly simplistic concept has been revised in recent years, as the effects of MMPs on ECM metabolism are not only of destructive character, but includes as well reparative functions, depending on the phase of MS. MMP-9 is involved in oligodendrocyte regrowth [203], while MMP-7 cleaves remyelination-impairing fibronectin to allow remyelination, and the authors speculate that a too low MMP-7 level contributes to the persistence of demyelination in chronic MS lesions and prevents oligodendrocyte maturation [204]. A further function of MMPs for synaptogenesis has been recognized [205, 206]. These findings are relevant for the concept how MMP inhibition could be used as therapeutic approach in MS. When leucocyte extravasation into lesions has ceased after re-establishment of the BBB, microglia are major sources of MMPs in the CNS. However, it is not known whether or when microglial activation is contributing to active progression in MS, or is actually a beneficial reaction to preserve tissue integrity. Hence, it is not clear at which time point and for how long therapeutic MMP inhibition could be beneficial in MS.
MMP inhibitors as treatment options for MS
Based on the evidence concerning the role of MMPs in BBB opening and lesion formation, MMP inhibitors (MMPIs) were thought to be an ideal class of compounds for MS therapy in the late 1990s. The development of specific MMPIs for oncology was followed with great interest by the neurology field. In vitro models of metastasis showed dramatic reduction of dissemination and growth of tumor cells, which were thought to be transferable to suppression of BBB transmigration of blood-derived leukocytes in MS. This was bolstered by the observation that current therapies such as IFNβ, or later natalizumab [207] downregulate MMPs as part of their mode of action. The hypothesis that the therapeutic effect of IFNβ may be mediated via its suppressive effect on MMP-9 expression in T cells was further corroborated by a reduction of their migration across an artificial BBB [208, 209].
A number of hydroxamic acid inhibitor type compounds, which inactivate the enzymatic activity of MMPs by binding to the catalytic zinc of MMPs, have led to disease severity attenuation in EAE [210,211,212]. Similarly, such compounds reduced the capacity of T cells to migrate across an in vitro BBB model [213]. However, for several reasons clinical studies have never started. In oncology, the initially promising preclinical results with MMPIs were not confirmed in clinical trials. The same was the case in rheumatoid arthritis. Additionally, depending on their enzymatic target profile, adverse events and specific signs of toxicity were recognized during chronic dosing, which precluded the intended long-term use as maintenance therapy in MS (for review see [214]). Evidence for the role of MMPs in tissue repair in subacute and stable phases of the disease suggests that long-term MMP inhibition could have detrimental effects. The intent to overcome this by targeting only ‘bad’ MMPs via hyper-selective substrate profiles fell short on the basis that tissue destruction and repair can be mediated by the very same MMPs (e.g., MMP-7 and -9) [204], depending on the acuity of disease at a given time point.
There is, however, one example of translation of the knowledge on the role of MMPs in MS. Minocycline, a tetracycline antibiotic that exerts broad-spectrum inhibition of MMPs, demonstrated in two phase 2 studies in MS efficacy in reducing gadolinium-enhancing lesions [215, 216], which encouraged the study group to execute a phase 3 clinical trial [217] with 142 patients with a first MS attack. Clinical and MRI findings suggested that minocycline in comparison to placebo reduced the risk for patients after a first attack to reach the criteria for formal MS diagnosis within 6 months by 28% (p = 0.006). The site of primary action of minocycline is likely in the ‘periphery’, i.e., the immune system outside the CNS, while it is not known which MMP’s inhibition is responsible for the clinical effect. Another potential mode of action apart from direct inhibition of enzymatic activity of several MMPs is interference with their activation. EMMPRIN (extracellular matrix metalloproteinase inducer, CD147) is a membrane glycoprotein of the immunoglobulin superfamily whose levels are reduced in T cells by minocycline [218]. Thus, minocycline treatment likely reduces the activity and secondarily the release and production of MMPs resulting in diminished leukocyte extravasation of leukocytes into the CNS. The Canadian study group that has run all minocycline trials in MS so far is preparing currently for another trial (Yong VW, personal communication).
Severity and frequency of relapses are important determinants for the long-term outcome of MS. Steroids are the current standard for intervention therapy to attenuate acute exacerbations in MS; however, their effect on the disability development is very limited. More powerful compounds for this situation represent a high unmet medical need. The effect of hydroxamic acid type MMPIs in EAE is strong pre-clinical evidence for their use in acute exacerbations in MS. Broad-spectrum MMPIs are the obvious drug candidates here, given the array of MMPs upregulated in this condition, as they have been shown to be free of side effects for a dosing period of several days [219]. Despite Health Authorities have defined the development path for the development of compounds for relapse therapy [220], the pharmaceutical industry has so far not engaged in such development activities. However, this may be an opportunity for a ‘second life’ for this class of candidate drugs.
Conclusion
Metalloproteinases are mediators of neuroinflammatory processes upon neuroinfections and in MS. During these neuroinflammatory diseases, metalloproteinases regulate BBB breakdown, neutrophil infiltration and cytokine signaling. Metalloproteinase inhibitors have experimentally been shown to decrease neuroinflammation and brain damage in diseases with excessive neuroinflammation as a common denominator, thereby improving the outcome of such diseases. Nevertheless, timing of metalloproteinase inhibition appears to be critical to effectively downmodulate the cascade of pathophysiological processes leading to brain damage during PM. On the other hand, metalloproteinases are also mediators of neuroregeneration and synaptogenesis, processes that are therapeutically not intended to be inhibited. As this critical role of metalloproteinases in neuronal repair mechanisms and regeneration was only lately recognized, the original idea of chronic MMP inhibition needs to be conceptually revised. Recently accumulated research urges for a second chance of MMPIs, which—when correctly applied and dosed—harbor the potential to improve many different neuroinflammatory diseases.
Abbreviations
- ADAM:
-
A disintegrin and metalloproteinase
- AJ:
-
Adherens junction
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- EAE:
-
Experimental autoimmune encephalitis
- EBV:
-
Epstein–Barr virus
- ECM:
-
Extracellular matrix
- HSE:
-
Herpes-simplex virus
- JEV:
-
Japanese encephalitis virus
- MAV-1:
-
Mouse adenovirus type-1
- MBP:
-
Myelin basic protein
- MMP:
-
Matrix metalloproteinase
- MMPI:
-
MMP inhibitor
- MS:
-
Multiple sclerosis
- PM:
-
Pneumococcal meningitis
- PPMS:
-
Primary progressive MS
- ROS:
-
Reactive oxygen species
- RRMS:
-
Relapsing–remitting MS
- SPMS:
-
Secondary progressive MS
- TACE:
-
TNF-α-converting enzyme
- TBM:
-
Tuberculous meningitis
- TIMP:
-
Tissue inhibitor of metalloproteinase
- TJ:
-
Tight junction
- WNV:
-
West Nile virus
References
Yong VW (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 6:931–944. https://doi.org/10.1038/nrn1807
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516. https://doi.org/10.1146/annurev.cellbio.17.1.463
Mott JD, Werb Z (2004) Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564. https://doi.org/10.1016/j.ceb.2004.07.010
McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol 13:534–540
Parks WC, Wilson CL, López-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4:617–629. https://doi.org/10.1038/nri1418
White JM (2003) ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol 15:598–606
Yong VW, Power C, Forsyth P, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511. https://doi.org/10.1038/35081571
Overall CM, López-Otín C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2:657–672. https://doi.org/10.1038/nrc884
Van Wart HE, Birkedal-Hansen H (1990) The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA 87:5578–5582
Weiss SJ, Peppin G, Ortiz X et al (1985) Oxidative autoactivation of latent collagenase by human neutrophils. Science 227:747–749
Peppin GJ, Weiss SJ (1986) Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci USA 83:4322–4326
Fu X, Kassim SY, Parks WC, Heinecke JW (2001) Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem 276:41279–41287. https://doi.org/10.1074/jbc.M106958200
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Murphy G, Knäuper V, Lee M-H et al (2003) Role of TIMPs (tissue inhibitors of metalloproteinases) in pericellular proteolysis: the specificity is in the detail. Biochem Soc Symp 70:65–80
Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665. https://doi.org/10.1038/nri3499
Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M (1990) Collagenase-induced intracerebral hemorrhage in rats. Stroke 21:801–807
Rosenberg GA, Kornfeld M, Estrada E et al (1992) TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res 576:203–207. https://doi.org/10.1016/0006-8993(92)90681-X
Baranger K, Rivera S, Liechti FD et al (2014) Endogenous and synthetic MMP inhibitors in CNS physiopathology. Prog Brain Res 214:313–351
Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG (1995) Tumor necrosis factor-α-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res 703:151–155. https://doi.org/10.1016/0006-8993(95)01089-0
Mun-Bryce S, Lukes A, Wallace J et al (2002) Stromelysin-1 and gelatinase A are upregulated before TNF-alpha in LPS-stimulated neuroinflammation. Brain Res 933:42–49
Mun-Bryce S, Rosenberg GA (1998) Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol 274:R1203–R1211
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. https://doi.org/10.3389/fneur.2013.00032
Sellner J, Leib SL (2006) In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol Dis 21:647–656. https://doi.org/10.1016/j.nbd.2005.09.007
Rosenberg GA, Estrada EY, Dencoff JE (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29:2189–2195
Yang Y, Estrada EY, Thompson JF et al (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709. https://doi.org/10.1038/sj.jcbfm.9600375
Chen F, Ohashi N, Li W et al (2009) Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 50:1914–1923. https://doi.org/10.1002/hep.23203
McColl BW, Rothwell NJ, Allan SM (2008) Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 28:9451–9462. https://doi.org/10.1523/JNEUROSCI.2674-08.2008
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7:243–253
Gidday JM, Gasche YG, Copin J-C et al (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Circ Physiol 289:H558–H568. https://doi.org/10.1152/ajpheart.01275.2004
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87:779–789. https://doi.org/10.1189/jlb.1109766
Justicia C, Panés J, Solé S et al (2003) neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab 23:1430–1440. https://doi.org/10.1097/01.WCB.0000090680.07515.C8
Meli DN, Christen S, Leib SL (2003) Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J Infect Dis 187:1411–1415. https://doi.org/10.1086/374644
Opdenakker G, Van den Steen PE, Van Damme J (2001) Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 22:571–579
Masure S, Proost P, Van Damme J, Opdenakker G (1991) Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem 198:391–398
Leppert D, Leib SL, Grygar C et al (2000) Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis 31:80–84. https://doi.org/10.1086/313922
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41:271–290. https://doi.org/10.1007/s00726-010-0689-x
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood–brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23:87–96. https://doi.org/10.1016/j.nbd.2006.02.006
Ueno M, Wu B, Nishiyama A et al (2009) The expression of matrix metalloproteinase-13 is increased in vessels with blood–brain barrier impairment in a stroke-prone hypertensive model. Hypertens Res 32:332–338. https://doi.org/10.1038/hr.2009.26
Cuadrado E, Rosell A, Penalba A et al (2009) Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res 8:3191–3197. https://doi.org/10.1021/pr801012x
Cuadrado E, Rosell A, Borrell-Pagès M et al (2009) Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J Cereb Blood Flow Metab 29:398–410. https://doi.org/10.1038/jcbfm.2008.130
Kieseier BC, Paul R, Koedel U et al (1999) Differential expression of matrix metalloproteinases in bacterial meningitis. Brain 122(Pt 8):1579–1587
Cui D, Arima M, Takubo K et al (2015) ADAM12 and ADAM17 are essential molecules for hypoxia-induced impairment of neural vascular barrier function. Sci Rep 5:12796. https://doi.org/10.1038/srep12796
Inoshima I, Inoshima N, Wilke GA et al (2011) A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17:1310–1314. https://doi.org/10.1038/nm.2451
Ponnuchamy B, Khalil RA (2008) Role of ADAMs in endothelial cell permeability: cadherin shedding and leukocyte rolling. Circ Res 102:1139–1142. https://doi.org/10.1161/CIRCRESAHA.108.177394
Gross J, Lapiere CM (1962) Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48:1014–1022
Van den Steen PE, Proost P, Wuyts A et al (2000) Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96:2673–2681
McQuibban GA, Gong JH, Tam EM et al (2000) Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289:1202–1206
McQuibban GA, Gong J-H, Wong JP et al (2002) Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100:1160–1167
McQuibban GA, Butler GS, Gong J-H et al (2001) Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem 276:43503–43508. https://doi.org/10.1074/jbc.M107736200
Zhang K, McQuibban GA, Silva C et al (2003) HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci 6:1064–1071. https://doi.org/10.1038/nn1127
Song J, Wu C, Zhang X, Sorokin LM (2013) In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1-induced peritonitis. J Immunol 190:401–410. https://doi.org/10.4049/jimmunol.1202286
Tester AM, Cox JH, Connor AR et al (2007) LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS One 2:e312. https://doi.org/10.1371/journal.pone.0000312
Afonso PV, McCann CP, Kapnick SM, Parent CA (2013) Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood 121:1644–1650. https://doi.org/10.1182/blood-2012-08-451575
Van Den Steen PE, Wuyts A, Husson SJ et al (2003) Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem 270:3739–3749
Khandaker MH, Mitchell G, Xu L et al (1999) Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR55 and CXCR55 chemokine receptor expression. Blood 93:2173–2185
Li Q, Park PW, Wilson CL, Parks WC (2002) Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111:635–646
Corry DB, Rishi K, Kanellis J et al (2002) Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 3:347–353. https://doi.org/10.1038/ni773
Corry DB, Kiss A, Song L-Z et al (2004) Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J 18:995–997. https://doi.org/10.1096/fj.03-1412fje
Thornberry NA, Bull HG, Calaycay JR et al (1992) A novel heterodimeric cysteine protease is required for interleukin-1βprocessing in monocytes. Nature 356:768–774. https://doi.org/10.1038/356768a0
Kostura MJ, Tocci MJ, Limjuco G et al (1989) Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci USA 86:5227–5231
Fantuzzi G, Ku G, Harding MW et al (1997) Response to local inflammation of IL-1 beta-converting enzyme-deficient mice. J Immunol 158:1818–1824
Schönbeck U, Mach F, Libby P (1998) Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161:3340–3346
Moss ML, Jin S-LC, Milla ME et al (1997) Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385:733–736. https://doi.org/10.1038/385733a0
Black RA, Rauch CT, Kozlosky CJ et al (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729–733. https://doi.org/10.1038/385729a0
Mohan MJ, Seaton T, Mitchell J et al (2002) The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry 41:9462–9469
Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14:163–176
Karsdal MA, Larsen L, Engsig MT et al (2002) Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 277:44061–44067. https://doi.org/10.1074/jbc.M207205200
Maeda S, Dean DD, Gomez R et al (2002) The first stage of transforming growth factor β1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3). Calcif Tissue Int 70:54–65. https://doi.org/10.1007/s002230010032
Munger JS, Huang X, Kawakatsu H et al (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328
Shull MM, Ormsby I, Kier AB et al (1992) Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359:693–699. https://doi.org/10.1038/359693a0
Gearing AJH, Beckett P, Christodoulou M et al (1994) Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370:555–557. https://doi.org/10.1038/370555a0
Grell M, Douni E, Wajant H et al (1995) The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83:793–802
Bell JH, Herrera AH, Li Y, Walcheck B (2007) Role of ADAM17 in the ectodomain shedding of TNF-and its receptors by neutrophils and macrophages. J Leukoc Biol 82:173–176. https://doi.org/10.1189/jlb.0307193
Peschon JJ, Slack JL, Reddy P et al (1998) An essential role for ectodomain shedding in mammalian development. Science 282:1281–1284
Horiuchi K, Kimura T, Miyamoto T et al (2007) Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol 179:2686–2689
Smookler DS, Mohammed FF, Kassiri Z et al (2006) Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol 176:721–725
Garbers C, Jänner N, Chalaris A et al (2011) Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem 286:14804–14811. https://doi.org/10.1074/jbc.M111.229393
Briso EM, Dienz O, Rincon M (2008) Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol 180:7102–7106
Smalley DM, Ley K (2005) L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med 9:255–266
Tang J, Zarbock A, Gomez I et al (2011) Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood 118:786–794. https://doi.org/10.1182/blood-2010-11-321406
Long C, Hosseinkhani MR, Wang Y et al (2012) ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J Leukoc Biol 92:667–672. https://doi.org/10.1189/jlb.0312112
Sahin U, Weskamp G, Kelly K et al (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164:769–779. https://doi.org/10.1083/jcb.200307137
Chalaris A, Adam N, Sina C et al (2010) Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med 207:1617–1624. https://doi.org/10.1084/jem.20092366
Brou C, Logeat F, Gupta N et al (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5:207–216
van Tetering G, van Diest P, Verlaan I et al (2009) Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J Biol Chem 284:31018–31027. https://doi.org/10.1074/jbc.M109.006775
Murthy A, Shao YW, Narala SR et al (2012) Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 36:105–119. https://doi.org/10.1016/j.immuni.2012.01.005
Wang Y, Wu J, Newton R et al (2013) ADAM17 cleaves CD16b (FcγRIIIb) in human neutrophils. Biochim Biophys Acta 1833:680–685. https://doi.org/10.1016/j.bbamcr.2012.11.027
Moncada-Pazos A, Obaya AJ, Llamazares M et al (2012) ADAMTS-12 metalloprotease is necessary for normal inflammatory response. J Biol Chem 287:39554–39563. https://doi.org/10.1074/jbc.M112.408625
Tremblay M-E, Stevens B, Sierra A et al (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16069. https://doi.org/10.1523/JNEUROSCI.4158-11.2011
Graeber MB, Streit WJ (2010) Microglia: biology and pathology. Acta Neuropathol 119:89–105. https://doi.org/10.1007/s00401-009-0622-0
Cherry JD, Olschowka JA, O’Banion M (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
Boche D, Perry VH, Nicoll JAR (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39:3–18. https://doi.org/10.1111/nan.12011
Rosenberg GA (2002) Matrix metalloproteinases in neuroinflammation. Glia 39:279–291. https://doi.org/10.1002/glia.10108
Nuttall RK, Silva C, Hader W et al (2007) Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia 55:516–526. https://doi.org/10.1002/glia.20478
Huang W-C, Sala-Newby GB, Susana A et al (2012) Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One 7:e42507. https://doi.org/10.1371/journal.pone.0042507
Kanazawa M, Ninomiya I, Hatakeyama M et al (2017) Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 18:2135. https://doi.org/10.3390/ijms18102135
Kanazawa M, Miura M, Toriyabe M et al (2017) Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci Rep 7:42582. https://doi.org/10.1038/srep42582
Lee S-R, Kim H-Y, Rogowska J et al (2006) Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 26:3491–3495. https://doi.org/10.1523/JNEUROSCI.4085-05.2006
Wang L, Zhang ZG, Zhang RL et al (2006) Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci 26:5996–6003. https://doi.org/10.1523/JNEUROSCI.5380-05.2006
Zhao B-Q, Wang S, Kim H-Y et al (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12:441–445. https://doi.org/10.1038/nm1387
Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158:983–994. https://doi.org/10.1016/j.neuroscience.2008.06.025
Rosell A, Lo E (2008) Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 8:82–89. https://doi.org/10.1016/j.coph.2007.12.001
Sood RR, Taheri S, Candelario-Jalil E et al (2008) Early beneficial effect of matrix metalloproteinase inhibition on blood—brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J Cereb Blood Flow Metab 28:431–438. https://doi.org/10.1038/sj.jcbfm.9600534
Agyeman P, Grandgirard D, Leib SL (2017) Neuroinflammation in bacterial meningitis. The blood brain barrier and inflammation. Springer International Publishing, Cham, pp 213–252
Agyeman P, Grandgirard D, Leib S (2014) Pathogenesis and pathophysiology of bacterial infections. In: Scheld M, Whitley RJ, Marra CM (eds) Infections of the central nervous system. Lippincott Williams & Wilkins, Philadelphia, PA, USA, pp 341–364
van de Beek D, Cabellos C, Dzupova O et al (2016) ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22:S37–S62. https://doi.org/10.1016/j.cmi.2016.01.007
Koedel U, Scheld WM, Pfister H-W (2002) Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2:721–736
Edmond K, Clark A, Korczak VS et al (2010) Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 10:317–328. https://doi.org/10.1016/S1473-3099(10)70048-7
Brouwer MC, Tunkel AR, van de Beek D (2010) Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 23:467–492. https://doi.org/10.1128/CMR.00070-09
Wellmer A, Noeske C, Gerber J et al (2000) Spatial memory and learning deficits after experimental pneumococcal meningitis in mice. Neurosci Lett 296:137–140
Loeffler JM, Ringer R, Hablützel M et al (2001) The free radical scavenger α-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J Infect Dis 183:247–252. https://doi.org/10.1086/317921
Nau R, Brück W (2002) Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci 25:38–45
Leib SL, Heimgartner C, Bifrare Y-D et al (2003) Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr Res 54:353–357. https://doi.org/10.1203/01.PDR.0000079185.67878.72
Nau R, Soto A, Bruck W (1999) Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol 58:265–274. https://doi.org/10.1097/00005072-199903000-00006
Gerber J, Nau R (2010) Mechanisms of injury in bacterial meningitis. Curr Opin Neurol 23:312–318. https://doi.org/10.1097/WCO.0b013e32833950dd
Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D (2011) Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 24:557–591. https://doi.org/10.1128/CMR.00008-11
Mitchell L, Smith SH, Braun JS et al (2004) Dual phases of apoptosis in pneumococcal meningitis. J Infect Dis 190:2039–2046. https://doi.org/10.1086/425520
Gianinazzi C, Grandgirard D, Imboden H et al (2003) Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol 105:499–507. https://doi.org/10.1007/s00401-003-0672-7
Iliev AI, Stringaris AK, Nau R, Neumann H (2003) Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9). FASEB J 18:412–414. https://doi.org/10.1096/fj.03-0670fje
Marques CP, Cheeran MC-J, Palmquist JM et al (2008) Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J Neurovirol 14:229–238. https://doi.org/10.1080/13550280802093927
Perny M, Solyga M, Grandgirard D et al (2017) Streptococcus pneumoniae-induced ototoxicity in organ of Corti explant cultures. Hear Res 350:100–109. https://doi.org/10.1016/j.heares.2017.04.012
Perny M, Roccio M, Grandgirard D et al (2016) The severity of infection determines the localization of damage and extent of sensorineural hearing loss in experimental pneumococcal meningitis. J Neurosci 36:7740–7749. https://doi.org/10.1523/JNEUROSCI.0554-16.2016
Klein M, Koedel U, Pfister H-W, Kastenbauer S (2003) Morphological correlates of acute and permanent hearing loss during experimental pneumococcal meningitis. Brain Pathol 13:123–132. https://doi.org/10.1111/j.1750-3639.2003.tb00012.x
van de Beek D, de Gans J, Spanjaard L et al (2004) Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 351:1849–1859. https://doi.org/10.1056/NEJMoa040845
Chandran A, Herbert H, Misurski D, Santosham M (2011) Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J 30:3–6. https://doi.org/10.1097/INF.0b013e3181ef25f7
van Furth AM, Roord JJ, van Furth R (1996) Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun 64:4883–4890
Leib SL, Clements JM, Lindberg RLP et al (2001) Inhibition of matrix metalloproteinases and tumour necrosis factor α converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734–1742. https://doi.org/10.1093/brain/124.9.1734
Leib SL, Leppert D, Clements J, Täuber MG (2000) Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 68:615–620
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216. https://doi.org/10.1016/S1474-4422(09)70016-X
Leppert D, Lindberg RLP, Kappos L, Leib SL (2001) Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Rev 36:249–257. https://doi.org/10.1016/S0165-0173(01)00101-1
Schubert-Unkmeir A, Konrad C, Slanina H et al (2010) Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog 6:e1000874. https://doi.org/10.1371/journal.ppat.1000874
Meli D, Loeffler J, Baumann P et al (2004) In pneumococcal meningitis a novel water-soluble inhibitor of matrix metalloproteinases and TNF-α converting enzyme attenuates seizures and injury of the cerebral cortex. J Neuroimmunol 151:6–11. https://doi.org/10.1016/j.jneuroim.2004.01.026
Chandler S, Miller KM, Clements JM et al (1997) Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol 72:155–161
Asahi M, Wang X, Mori T et al (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732
Böttcher T, Spreer A, Azeh I et al (2003) Matrix metalloproteinase-9 deficiency impairs host defense mechanisms against Streptococcus pneumoniae in a mouse model of bacterial meningitis. Neurosci Lett 338:201–204. https://doi.org/10.1016/S0304-3940(02)01406-4
Gu Z, Cui J, Brown S et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25:6401–6408. https://doi.org/10.1523/JNEUROSCI.1563-05.2005
Guo Z, Sun X, He Z et al (2010) Role of matrix metalloproteinase-9 in apoptosis of hippocampal neurons in rats during early brain injury after subarachnoid hemorrhage. Neurol Sci 31:143–149. https://doi.org/10.1007/s10072-009-0192-x
Lindberg RLP, Sorsa T, Tervahartiala T et al (2006) Gelatinase B [matrix metalloproteinase (MMP)-9] and collagenases (MMP-8/-13) are upregulated in cerebrospinal fluid during aseptic and bacterial meningitis in children. Neuropathol Appl Neurobiol 32:304–317. https://doi.org/10.1111/j.1365-2990.2006.00729.x
Shapiro S, Miller A, Lahat N et al (2003) Expression of matrix metalloproteinases, sICAM-1 and IL-8 in CSF from children with meningitis. J Neurol Sci 206:43–48. https://doi.org/10.1016/S0022-510X(02)00317-9
Yushchenko M, Weber F, Mäder M et al (2000) Matrix metalloproteinase-9 (MMP-9) in human cerebrospinal fluid (CSF): elevated levels are primarily related to CSF cell count. J Neuroimmunol 110:244–251. https://doi.org/10.1016/S0165-5728(00)00339-8
Green JA, Thi Hong Chau T, Farrar JJ et al (2011) CNS infection, CSF matrix metalloproteinase concentrations, and clinical/laboratory features. Neurology 76:577–579. https://doi.org/10.1212/WNL.0b013e31820b7600
Tsai H-C, Shi M-H, Lee SS-J et al (2011) Expression of matrix metalloproteinases and their tissue inhibitors in the serum and cerebrospinal fluid of patients with meningitis. Clin Microbiol Infect 17:780–784. https://doi.org/10.1111/j.1469-0691.2010.03393.x
Koopmans MM, Brouwer MC, Geldhoff M et al (2014) Cerebrospinal fluid inflammatory markers in patients with Listeria monocytogenes meningitis. BBA Clin 1:44–51. https://doi.org/10.1016/J.BBACLI.2014.06.001
Roine I, Pelkonen T, Lauhio A et al (2015) Changes in MMP-9 and TIMP-1 concentrations in cerebrospinal fluid after 1 week of treatment of childhood bacterial meningitis. J Clin Microbiol 53:2340–2342. https://doi.org/10.1128/JCM.00714-15
Grandgirard D, Gäumann R, Coulibaly B et al (2013) The causative pathogen determines the inflammatory profile in cerebrospinal fluid and outcome in patients with bacterial meningitis. Mediat Inflamm 2013:1–12. https://doi.org/10.1155/2013/312476
Majeed S, Radotra BD, Sharma S (2016) Adjunctive role of MMP-9 inhibition along with conventional anti-tubercular drugs against experimental tuberculous meningitis. Int J Exp Pathol 97:230–237. https://doi.org/10.1111/iep.12191
Lee KY, Kim EH, Yang WS et al (2004) Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J Neurol Sci 220:73–78. https://doi.org/10.1016/j.jns.2004.02.008
Majeed S, Singh P, Sharma N, Sharma S (2016) Title: role of matrix metalloproteinase-9 in progression of tuberculous meningitis: a pilot study in patients at different stages of the disease. BMC Infect Dis 16:722. https://doi.org/10.1186/s12879-016-1953-9
Green JA, Tran CTH, Farrar JJ et al (2009) Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One 4:e7277. https://doi.org/10.1371/journal.pone.0007277
Thwaites GE, Bang ND, Dung NH et al (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351:1741–1751. https://doi.org/10.1056/NEJMoa040573
Spindler KR, Hsu T-H (2012) Viral disruption of the blood-brain barrier. Trends Microbiol 20:282–290. https://doi.org/10.1016/j.tim.2012.03.009
Kolb SA, Lahrtz F, Paul R et al (1998) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol 84:143–150
Tung W-H, Tsai H-W, Lee I-T et al (2010) Japanese encephalitis virus induces matrix metalloproteinase-9 in rat brain astrocytes via NF-κB signalling dependent on MAPKs and reactive oxygen species. Br J Pharmacol 161:1566–1583. https://doi.org/10.1111/j.1476-5381.2010.00982.x
Shukla V, Kumar Shakya A, Dhole TN, Misra UK (2012) Upregulated expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in BALB/c mouse brain challenged with Japanese encephalitis virus. NeuroImmunoModulation 19:241–254. https://doi.org/10.1159/000335182
Sellner J, Simon F, Meyding-Lamade U, Leib SL (2006) Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res 1125:155–162. https://doi.org/10.1016/j.brainres.2006.09.093
Verma S, Kumar M, Gurjav U et al (2010) Reversal of West Nile virus-induced blood–brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397:130–138. https://doi.org/10.1016/j.virol.2009.10.036
Wang P, Dai J, Bai F et al (2008) Matrix metalloproteinase 9 facilitates West Nile Virus entry into the brain. J Virol 82:8978–8985. https://doi.org/10.1128/JVI.00314-08
Eugenin EA, Osiecki K, Lopez L et al (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106. https://doi.org/10.1523/JNEUROSCI.3863-05.2006
Louboutin J-P, Agrawal L, Reyes BAS et al (2010) HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol 69:801–816. https://doi.org/10.1097/NEN.0b013e3181e8c96f
Conant K, McArthur JC, Griffin DE et al (1999) Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol 46:391–398
Liuzzi GM, Mastroianni CM, Santacroce MP et al (2000) Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J Neurovirol 6:156–163
Tirumuru N, Pretto CD, Castro Jorge LA, Spindler KR (2016) Mouse adenovirus type 1 early region 1A effects on the blood-brain barrier. mSphere 1:e00079-16. https://doi.org/10.1128/mSphere.00079-16
Ashley SL, Pretto CD, Stier MT et al (2017) Matrix metalloproteinase activity in infections by an encephalitic virus, mouse adenovirus type 1. J Virol 91:e01412-16. https://doi.org/10.1128/JVI.01412-16
Gralinski LE, Ashley SL, Dixon SD, Spindler KR (2009) Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol 83:9398–9410. https://doi.org/10.1128/JVI.00954-09
Zhou J, Marten NW, Bergmann CC et al (2005) Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J Virol 79:4764–4773. https://doi.org/10.1128/JVI.79.8.4764-4773.2005
Zhou J, Stohlman SA, Atkinson R et al (2002) Matrix metalloproteinase expression correlates with virulence following neurotropic mouse hepatitis virus infection. J Virol 76:7374–7384
Liechti FD, Grandgirard D, Leppert D, Leib SL (2014) Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infect Immun 82:1710–1718. https://doi.org/10.1128/IAI.00073-14
Liechti FD, Bächtold F, Grandgirard D et al (2015) The matrix metalloproteinase inhibitor RS-130830 attenuates brain injury in experimental pneumococcal meningitis. J Neuroinflamm 12:43. https://doi.org/10.1186/s12974-015-0257-0
Ricci S, Grandgirard D, Wenzel M et al (2014) Inhibition of matrix metalloproteinases attenuates brain damage in experimental meningococcal meningitis. BMC Infect Dis 14:726. https://doi.org/10.1186/s12879-014-0726-6
Echchannaoui H, Leib SL, Neumann U, Landmann RM (2007) Adjuvant TACE inhibitor treatment improves the outcome of TLR2−/−-mice with experimental pneumococcal meningitis. BMC Infect Dis 7:25. https://doi.org/10.1186/1471-2334-7-25
Rosenberg GA, Estrada EY, Mobashery S (2007) Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood–brain barrier opening in rodents: differences in response based on strains and solvents. Brain Res 1133:186–192. https://doi.org/10.1016/j.brainres.2006.11.041
Paul R, Lorenzl S, Koedel U et al (1998) Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann Neurol 44:592–600. https://doi.org/10.1002/ana.410440404
Meli DN, Coimbra RS, Erhart DG et al (2006) Doxycycline reduces mortality and injury to the brain and cochlea in experimental pneumococcal meningitis. Infect Immun 74:3890–3896. https://doi.org/10.1128/IAI.01949-05
Muri L, Grandgirard D, Buri M et al (2018) Combined effect of non-bacteriolytic antibiotic and inhibition of matrix metalloproteinases prevents brain injury and preserves learning, memory and hearing function in experimental paediatric pneumococcal meningitis. J Neuroinflamm 15:233. https://doi.org/10.1186/s12974-018-1272-8
Grandgirard D, Steiner O, Täuber MG, Leib SL (2007) An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol 114:609–617. https://doi.org/10.1007/s00401-007-0304-8
Bifrare Y-D, Gianinazzi C, Imboden H et al (2003) Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 13:481–488. https://doi.org/10.1002/hipo.10142
Liu X, Han Q (2014) Efficacy of GM6001 as an adjuvant to ceftriaxone in a neonatal rat model of Streptococcus pneumoniae meningitis. Acta Neurobiol Exp (Wars) 74:489–496
Barichello T, Generoso JS, Michelon CM et al (2014) Inhibition of matrix metalloproteinases-2 and -9 prevents cognitive impairment induced by pneumococcal meningitis in Wistar rats. Exp Biol Med (Maywood) 239:225–231. https://doi.org/10.1177/1535370213508354
Gerber J, Böttcher T, Hahn M et al (2004) Increased mortality and spatial memory deficits in TNF-α-deficient mice in ceftriaxone-treated experimental pneumococcal meningitis. Neurobiol Dis 16:133–138. https://doi.org/10.1016/J.NBD.2004.01.013
Thompson AJ, Baranzini SE, Geurts J et al (2018) Multiple sclerosis. Lancet 391:1622–1636. https://doi.org/10.1016/S0140-6736(18)30481-1
Dobson R, Giovannoni G (2018) Multiple sclerosis—a review. Eur J Neurol. https://doi.org/10.1111/ene.13819
Kutzelnigg A, Lucchinetti CF, Stadelmann C et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712. https://doi.org/10.1093/brain/awh641
Trapp BD, Vignos M, Dudman J et al (2018) Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol 17:870–884. https://doi.org/10.1016/S1474-4422(18)30245-X
Greenfield AL, Hauser SL (2018) B-cell therapy for multiple sclerosis: entering an era. Ann Neurol 83:13–26. https://doi.org/10.1002/ana.25119
Waubant E, Goodkin DE, Gee L et al (1999) Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology 53:1397–1401
Anthony DC, Ferguson B, Matyzak MK et al (1997) Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol 23:406–415
Cossins JA, Clements JM, Ford J et al (1997) Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol 94:590–598
Maeda A, Sobel RA (1996) Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol 55:300–309
Lindberg RL, De Groot CJ, Montagne L et al (2001) The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain 124:1743–1753
Kieseier BC, Kiefer R, Clements JM et al (1998) Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain 121(Pt 1):159–166
Dubois B, Masure S, Hurtenbach U et al (1999) Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest 104:1507–1515. https://doi.org/10.1172/JCI6886
Anthony DC, Miller KM, Fearn S et al (1998) Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J Neuroimmunol 87:62–72
Newman TA, Woolley ST, Hughes PM et al (2001) T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: involvement of metalloproteinases. Brain 124:2203–2214. https://doi.org/10.1093/brain/124.11.2203
Sellebjerg F, Börnsen L, Ammitzbøll C et al (2017) Defining active progressive multiple sclerosis. Mult Scler J 23:1727–1735. https://doi.org/10.1177/1352458517726592
Leppert D, Ford J, Stabler G et al (1998) Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain 121(Pt 12):2327–2334
Gijbels K, Masure S, Carton H, Opdenakker G (1992) Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol 41:29–34
Lee MA, Palace J, Stabler G et al (1999) Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain 122(Pt 2):191–197
Waubant E, Goodkin D, Bostrom A et al (2003) IFNbeta lowers MMP-9/TIMP-1 ratio, which predicts new enhancing lesions in patients with SPMS. Neurology 60:52–57
Rosenberg GA, Dencoff JE, Correa N et al (1996) Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology 46:1626–1632
Gilli F, Bertolotto A, Sala A et al (2004) Neutralizing antibodies against IFN- in multiple sclerosis: antagonization of IFN- mediated suppression of MMPs. Brain 127:259–268. https://doi.org/10.1093/brain/awh028
Bar-Or A, Nuttall RK, Duddy M et al (2003) Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 126:2738–2749. https://doi.org/10.1093/brain/awg285
Kouwenhoven M, Özenci V, Gomes A et al (2001) Multiple sclerosis: elevated expression of matrix metalloproteinases in blood monocytes. J Autoimmun 16:463–470. https://doi.org/10.1006/jaut.2001.0505
Oh LY, Larsen PH, Krekoski CA et al (1999) Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci 19:8464–8475
Wang P, Gorter RP, de Jonge JC et al (2018) MMP7 cleaves remyelination-impairing fibronectin aggregates and its expression is reduced in chronic multiple sclerosis lesions. Glia 66:1625–1643. https://doi.org/10.1002/glia.23328
Dear ML, Shilts J, Broadie K (2017) Neuronal activity drives FMRP- and HSPG-dependent matrix metalloproteinase function required for rapid synaptogenesis. Sci Signal 10:eaan3181. https://doi.org/10.1126/scisignal.aan3181
Reinhard SM, Razak K, Ethell IM (2015) A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front Cell Neurosci 9:280. https://doi.org/10.3389/fncel.2015.00280
Romme Christensen J, Ratzer R, Bornsen L et al (2014) Natalizumab in progressive MS: results of an open-label, phase 2A, proof-of-concept trial. Neurology 82:1499–1507. https://doi.org/10.1212/WNL.0000000000000361
Stüve O, Dooley NP, Uhm JH et al (1996) Interferon β-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol 40:853–863. https://doi.org/10.1002/ana.410400607
Leppert D, Waubant E, Bürk MR et al (1996) Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: a possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol 40:846–852. https://doi.org/10.1002/ana.410400606
Clements JM, Cossins JA, Wells GM et al (1997) Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol 74:85–94
Hewson AK, Smith T, Leonard JP, Cuzner ML (1995) Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res 44:345–349
Gijbels K, Galardy RE, Steinman L (1994) Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest 94:2177–2182. https://doi.org/10.1172/JCI117578
Leppert D, Waubant E, Galardy R et al (1995) T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 154:4379–4389
Keystone E (2002) Treatments no longer in development for rheumatoid arthritis. Ann Rheum Dis 61(Suppl 2):ii43–ii45. https://doi.org/10.1136/ARD.61.SUPPL_2.II43
Metz L, Li D, Traboulsee A et al (2009) Glatiramer acetate in combination with minocycline in patients with relapsing—remitting multiple sclerosis: results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult Scler J 15:1183–1194. https://doi.org/10.1177/1352458509106779
Metz LM, Zhang Y, Yeung M et al (2004) Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 55:756. https://doi.org/10.1002/ana.20111
Metz LM, Li DKB, Traboulsee AL et al (2017) Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med 376:2122–2133. https://doi.org/10.1056/NEJMoa1608889
Hahn JN, Kaushik DK, Mishra MK et al (2016) Impact of minocycline on extracellular matrix metalloproteinase inducer, a factor implicated in multiple sclerosis immunopathogenesis. J Immunol 197:3850–3860. https://doi.org/10.4049/jimmunol.1600436
Hemmings FJ, Farhan M, Rowland J et al (2001) Tolerability and pharmacokinetics of the collagenase-selective inhibitor Trocade in patients with rheumatoid arthritis. Rheumatology (Oxford) 40:537–543
European Medicines Agency (2015) Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. Committee for medicinal products for human use (CHMP). EMA/CHMP/771815/2011, Rev 2. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf. Accessed 4 June 2019
Acknowledgements
We thank Daria Baumgartner for her excellent help in designing the summary figure. This work was supported by a grant from the Swiss National Science Foundation (Grant 310030-162583).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muri, L., Leppert, D., Grandgirard, D. et al. MMPs and ADAMs in neurological infectious diseases and multiple sclerosis. Cell. Mol. Life Sci. 76, 3097–3116 (2019). https://doi.org/10.1007/s00018-019-03174-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03174-6