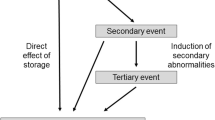
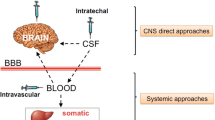
Abstract
Mucopolysaccharidoses (MPSs), which are inherited lysosomal storage disorders caused by the accumulation of undegraded glycosaminoglycans, can affect the central nervous system (CNS) and elicit cognitive and behavioral issues. Currently used enzyme replacement therapy methodologies often fail to adequately treat the manifestations of the disease in the CNS and other organs such as bone, cartilage, cornea, and heart. Targeted enzyme delivery systems (EDSs) can efficiently cross biological barriers such as blood–brain barrier and provide maximal therapeutic effects with minimal side effects, and hence, offer great clinical benefits over the currently used conventional enzyme replacement therapies. In this review, we provide comprehensive insights into MPSs and explore the clinical impacts of multimodal targeted EDSs.



Data were adapted with permission from a work published by Baldo et al. [101]

Data were adapted with permission from a work published by Sonoda et al. [108]
Similar content being viewed by others
References
Muenzer J (2014) Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol Genet Metab 111:63–72
Muenzer J (2011) Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 50:v4–v12
Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y (2010) Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function—a review. Clin Exp Ophthalmol 38:2–11
Cimaz R, La Torre F (2014) Mucopolysaccharidoses. Curr Rheumatol Rep 16:389
Kishnani PS, Dickson PI, Muldowney L, Lee JJ, Rosenberg A, Abichandani R, Bluestone JA, Burton BK, Dewey M, Freitas A (2016) Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol Genet Metab 117:66–83
Desnick R, Schuchman E (2012) Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu Rev Genom Hum Genet 13:307–335
Clarke LA (2011) Pathogenesis of skeletal and connective tissue involvement in the mucopolysaccharidoses: glycosaminoglycan storage is merely the instigator. Rheumatology (Oxford) 50:v13–v18
Shapiro EG, Jones SA, Escolar ML (2017) Developmental and behavioral aspects of mucopolysaccharidoses with brain manifestations—neurological signs and symptoms. Mol Genet Metab 122:1–7
Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF (2003) Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA 100:1902–1907
Rigante D, Cipolla C, Basile U, Gulli F, Savastano MC (2017) Overview of immune abnormalities in lysosomal storage disorders. Immunol Lett 188:79–85
Solomon M, Muro S (2017) Lysosomal enzyme replacement therapies: historical development, clinical outcomes, and future perspectives. Adv Drug Deliv Rev 118:109–134
Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S (2016) Identification and molecular characterization of genes coding pharmaceutically important enzymes from halo-thermo tolerant bacillus. Adv Pharm Bull 6:551
Valayannopoulos V, Wijburg FA (2011) Therapy for the mucopolysaccharidoses. Rheumatology (Oxford) 50:v49–v59
Pan D (2011) Cell-and gene-based therapeutic approaches for neurological deficits in mucopolysaccharidoses. Curr Pharm Biotechnol 12:884–896
Safary A, Khiavi MA, Mousavi R, Barar J, Rafi MA (2018) Enzyme replacement therapies: what is the best option? BioImpacts 8:153–157
Schuh RS, Baldo G, Teixeira HF (2016) Nanotechnology applied to treatment of mucopolysaccharidoses. Expert Opin Drug Deliv 13:1709–1718
Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE (1991) Replacement therapy for inherited enzyme deficiency—macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med 324:1464–1470
Barton NW, Furbish FS, Murray GJ, Garfield M, Brady RO (1990) Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci USA 87:1913–1916
Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, Parker C, Schiffmann R, Hill SC, Brady RO (1995) Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med 122:33–39
Khan KH (2013) Gene expression in mammalian cells and its applications. Adv Pharm Bull 3:257
Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S (2016) A strategy for soluble overexpression and biochemical characterization of halo-thermotolerant Bacillus laccase in modified E. coli. J Biotechnol 227:56–63
Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S (2019) Highly efficient novel recombinant l-asparaginase with no glutaminase activity from a new halo-thermotolerant Bacillus strain. BioImpacts 9:15–23
Kermode AR (2006) Plants as factories for production of biopharmaceutical and bioindustrial proteins: lessons from cell biology. Botany 84:679–694
He X, Haselhorst T, Von Itzstein M, Kolarich D, Packer NH, Gloster TM, Vocadlo DJ, Clarke LA, Qian Y, Kermode AR (2012) Production of α-l-iduronidase in maize for the potential treatment of a human lysosomal storage disease. Nat Commun 3:1062
Kim J, Park M, Kim D, Lee J, Maeng S, Cho S, Han Y, Ahn K, Jin D (2013) IgE-mediated anaphylaxis and allergic reactions to idursulfase in patients with Hunter syndrome. Allergy 68:796–802
Le SQ, Kan S-H, Clarke D, Sanghez V, Egeland M, Vondrak KN, Doherty TM, Vera MU, Iacovino M, Cooper JD (2018) A humoral immune response alters the distribution of enzyme replacement therapy in murine mucopolysaccharidosis type I. Mol Ther Methods Clin Dev 8:42–51
Coutinho MF, Prata MJ, Alves S (2012) Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction. Mol Genet Metab 105:542–550
Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H (2010) Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 99:26–33
Wraith JE, Beck M, Lane R, Van Der Ploeg A, Shapiro E, Xue Y, Kakkis ED, Guffon N (2007) Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human α-l-iduronidase (laronidase). Pediatrics 120:e37–e46
Banugaria SG, Prater SN, Ng Y-K, Kobori JA, Finkel RS, Ladda RL, Chen Y-T, Rosenberg AS, Kishnani PS (2011) The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 13:729
Calias P, Banks WA, Begley D, Scarpa M, Dickson P (2014) Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacol Ther 144:114–122
Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED (2008) Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Investig 118:2868–2876
Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M (2006) A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 8:465
Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED (2004) Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-l-iduronidase (laronidase). J Pediatr 144:581–588
Miebach E (2009) Management of infusion-related reactions to enzyme replacement therapy in a cohort of patients with mucopolysaccharidosis disorders. Int J Clin Pharmacol Ther 47:S100–S106
Barar J, Rafi MA, Pourseif MM, Omidi Y (2016) Blood–brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts 6:225
Fraldi A, Annunziata F, Lombardi A, Kaiser HJ, Medina DL, Spampanato C, Fedele AO, Polishchuk R, Sorrentino NC, Simons K (2010) Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J 29:3607–3620
Omidi Y, Gumbleton M (2005) Biological membranes and barriers. In: Mahato RI (ed) Biomaterials for delivery and targeting of proteins nucleic acids. CRC Press, Baco Raton, pp 232–274
Lajoie JM, Shusta EV (2015) Targeting receptor-mediated transport for delivery of biologics across the blood–brain barrier. Annu Rev Pharmacol Toxicol 55:613–631
Achord DT, Brot FE, Bell CE, Sly WS (1978) Human β-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell 15:269–278
Zhu Y, Li X, Schuchman EH, Desnick RJ, Cheng SH (2004) Dexamethasone-mediated up-regulation of the mannose receptor improves the delivery of recombinant glucocerebrosidase to Gaucher macrophages. J Pharmacol Exp Ther 308:705–711
Koeberl DD, Luo X, Sun B, McVie-Wylie A, Dai J, Li S, Banugaria SG, Chen Y-T, Bali DS (2011) Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol Genet Metab 103:107–112
Urayama A, Grubb JH, Sly WS, Banks WA (2008) Mannose 6-phosphate receptor—mediated transport of sulfamidase across the blood–brain barrier in the newborn mouse. Mol Ther 16:1261–1266
Rappaport J, Manthe RL, Garnacho C, Muro S (2015) Altered clathrin-independent endocytosis in type A Niemann-Pick disease cells and rescue by ICAM-1-targeted enzyme delivery. Mol Pharm 12:1366–1376
Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, Pavloff N, Sly WS (2005) Overcoming the blood–brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA 102:14777–14782
Calias P, Papisov M, Pan J, Savioli N, Belov V, Huang Y, Lotterhand J, Alessandrini M, Liu N, Fischman AJ (2012) CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS One 7:e30341
Vite CH, Wang P, Patel RT, Walton RM, Walkley SU, Sellers RS, Ellinwood NM, Cheng AS, White JT, O’Neill CA (2011) Biodistribution and pharmacodynamics of recombinant human alpha-l-iduronidase (rhIDU) in mucopolysaccharidosis type I-affected cats following multiple intrathecal administrations. Mol Genet Metab 103:268–274
Dickson P, Kaitila I, Harmatz P, Mlikotic A, Chen A, Victoroff A, Passage M, Madden J, Le S, Naylor D (2015) Data from subjects receiving intrathecal laronidase for cervical spinal stenosis due to mucopolysaccharidosis type I. Data Brief 5:71–76
Muenzer J, Hendriksz CJ, Fan Z, Vijayaraghavan S, Perry V, Santra S, Solanki GA, Mascelli MA, Pan L, Wang N (2016) A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med 18:73
Jones SA, Breen C, Heap F, Rust S, de Ruijter J, Tump E, Marchal JP, Pan L, Qiu Y, Chung J-K (2016) A phase 1/2 study of intrathecal heparan-N-sulfatase in patients with mucopolysaccharidosis IIIA. Mol Genet Metab 118:198–205
Nestrasil I, Shapiro E, Svatkova A, Dickson P, Chen A, Wakumoto A, Ahmed A, Stehel E, McNeil S, Gravance C, Maher E (2017) Intrathecal enzyme replacement therapy reverses cognitive decline in mucopolysaccharidosis type I. Am J Med Genet A 173:780–783
Scarpa M, Orchard PJ, Schulz A, Dickson PI, Haskins ME, Escolar ML, Giugliani R (2017) Treatment of brain disease in the mucopolysaccharidoses. Mol Genet Metab 122:25–34
Lampe C, Bellettato CM, Karabul N, Scarpa M (2013) Mucopolysaccharidoses and other lysosomal storage diseases. Rheum Dis Clin 39:431–455
Tomatsu S, Alméciga-Díaz CJ, Montaño AM, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E (2015) Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab 114:94–109
Muro S (2010) New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2:189–204
LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C, Sly WS (2004) Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci USA 101:3083–3088
Prince WS, McCormick LM, Wendt DJ, Fitzpatrick PA, Schwartz KL, Aguilera AI, Koppaka V, Christianson TM, Vellard MC, Pavloff N (2004) Lipoprotein receptor binding, cellular uptake, and lysosomal delivery of fusions between the receptor-associated protein (RAP) and α-l-iduronidase or acid α-glucosidase. J Biol Chem 279:35037–35046
Orii KO, Grubb JH, Vogler C, Levy B, Tan Y, Markova K, Davidson BL, Mao Q, Orii T, Kondo N (2005) Defining the pathway for Tat-mediated delivery of β-glucuronidase in cultured cells and MPS VII mice. Mol Ther 12:345–352
Giugliani R, Giugliani L, de Oliveira Poswar F, Donis KC, Dalla Corte A, Schmidt M, Boado RJ, Nestrasil I, Nguyen C, Chen S (2018) Neurocognitive and somatic stabilization in pediatric patients with severe mucopolysaccharidosis type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1–2 trial. Orphanet J Rare Dis 13:110
Barar J, Omidi Y (2013) Nanoparticles for ocular drug delivery. In: Kumar A, Mansour HM, Friedman A, Blough ER (eds) Nanomedicine in drug delivery. CRC Press, Baco Raton, pp 287–334
Khiavi MA, Safary A, Aghanejad A, Barar J, Rasta SH, Golchin A, Omidi Y, Somi MH (2019) Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells. Colloids Surf A Physicochem Eng Asp 572:333–344
Desnick RJ, Schuchman EH (2002) Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet 3:954
Tam VH, Sosa C, Liu R, Yao N, Priestley RD (2016) Nanomedicine as a non-invasive strategy for drug delivery across the blood–brain barrier. Int J Pharm 515:331–342
Desnick R, Fiddler M, Steger L, Cumming D, Dullum C, Thorpe S (1975) Enzyme replacement therapy-invivo fate of native, erythrocyte-entrapped and liposome-entrapped beta-glucuronidase. Pediatr Res 9:312
Samad A, Sultana Y, Aqil M (2007) Liposomal drug delivery systems: an update review. Curr Drug Deliv 4:297–305
Mayer FQ, Adorne MD, Bender EA, de Carvalho TG, Dilda AC, Beck RCR, Guterres SS, Giugliani R, Matte U, Pohlmann AR (2015) Laronidase-functionalized multiple-wall lipid-core nanocapsules: promising formulation for a more effective treatment of mucopolysaccharidosis type I. Pharm Res 32:941–954
Mooguee M, Omidi Y, Davaran S (2010) Synthesis and in vitro release of adriamycin from star-shaped poly(lactide-co-glycolide) nano- and microparticles. J Pharm Sci 99:3389–3397
Moogooee M, Ramezanzadeh H, Jasoori S, Omidi Y, Davaran S (2011) Synthesis and in vitro studies of cross-linked hydrogel nanoparticles containing amoxicillin. J Pharm Sci 100:1057–1066
Khosroushahi AY, Naderi-Manesh H, Yeganeh H, Barar J, Omidi Y (2012) Novel water-soluble polyurethane nanomicelles for cancer chemotherapy: physicochemical characterization and cellular activities. J Nanobiotechnol 10:2
Barzegar-Jalali M, Hanaee J, Omidi Y, Ghanbarzadeh S, Ziaee S, Bairami-Atashgah R, Adibkia K (2013) Preparation and evaluation of sustained release calcium alginate beads and matrix tablets of acetazolamide. Drug Res (Stuttg) 63:60–64
Shakoori Z, Ghanbari H, Omidi Y, Pashaiasl M, Akbarzadeh A, Jomeh Farsangi Z, Rezayat SM, Davaran S (2017) Fluorescent multi-responsive cross-linked P(N-isopropylacrylamide)-based nanocomposites for cisplatin delivery. Drug Dev Ind Pharm 43:1283–1291
Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y (2017) Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr Polym 172:130–141
Matthaiou E-I, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y (2014) Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomed 9:1855
Salvalaio M, Rigon L, Belletti D, D’Avanzo F, Pederzoli F, Ruozi B, Marin O, Vandelli MA, Forni F, Scarpa M (2016) Targeted polymeric nanoparticles for brain delivery of high molecular weight molecules in lysosomal storage disorders. PLoS One 11:e0156452
Papademetriou J, Garnacho C, Serrano D, Bhowmick T, Schuchman EH, Muro S (2013) Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J Inherit Metab Dis 36:467–477
Abuchowski A, Van Es T, Palczuk N, Davis F (1977) Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem 252:3578–3581
Dozier JK, Distefano MD (2015) Site-specific PEGylation of therapeutic proteins. Int J Mol Sci 16:25831–25864
Nischan N, Hackenberger CP (2014) Site-specific PEGylation of proteins: recent developments. J Org Chem 79:10727–10733
Fraga M, Bruxel F, Diel D, de Carvalho TG, Perez CA, Magalhães-Paniago R, Malachias Â, Oliveira MC, Matte U, Teixeira HF (2015) PEGylated cationic nanoemulsions can efficiently bind and transfect pIDUA in a mucopolysaccharidosis type I murine model. J Control Release 209:37–46
Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM (2008) Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 29:215–227
Wilson B (2009) Brain targeting PBCA nanoparticles and the blood–brain barrier. Nanomedicine 4:499–502
Sinha V, Bansal K, Kaushik R, Kumria R, Trehan A (2004) Poly-ε-caprolactone microspheres and nanospheres: an overview. Int J Pharm 278:1–23
Papademetriou I, Tsinas Z, Hsu J, Muro S (2014) Combination-targeting to multiple endothelial cell adhesion molecules modulates binding, endocytosis, and in vivo biodistribution of drug nanocarriers and their therapeutic cargoes. J Control Release 188:87–98
Serrano D, Muro S (2014) Endothelial cell adhesion molecules and drug delivery applications. In: Aranda-Espinoza H (ed) Mechanobiology of the endothelium. CRC Press, Boca Raton, pp 4100–32
Serrano D, Bhowmick T, Chadha R, Garnacho C, Muro S (2012) Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler Thromb Vasc Biol 32:1178–1185
Hsu J, Northrup L, Bhowmick T, Muro S (2012) Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: comparative performance of a strategy for three distinct lysosomal storage disorders. Nanomed Nanotechnol Biol Med 8:731–739
Hsu J, Serrano D, Bhowmick T, Kumar K, Shen Y, Kuo YC, Garnacho C, Muro S (2011) Enhanced endothelial delivery and biochemical effects of α-galactosidase by ICAM-1-targeted nanocarriers for Fabry disease. J Control Release 149:323–331
Hsu JB, Bhowmick T, Burks SR, Kao JP, Muro S (2014) Enhancing biodistribution of therapeutic enzymes in vivo by modulating surface coating and concentration of ICAM-1-targeted nanocarriers. J Biomed Nanotechnol 10:345–354
Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S (2008) Delivery of acid sphingomyelinase in normal and Niemann–Pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther 325:400–408
Wang G, Mostafa NZ, Incani V, Kucharski C, Uludağ H (2012) Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J Biomed Mater Res A 100:684–693
De Pasquale V, Sarogni P, Pistorio V, Cerulo G, Paladino S, Pavone LM (2018) Targeting heparan sulfate proteoglycans as a novel therapeutic strategy for mucopolysaccharidoses. Mol Ther Methods Clin Dev 10:8–16
Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z (2017) Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B 7:281–291
Barar J, Aghanejad A, Fathi M, Omidi Y (2016) Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts 6:49
Barar J, Javadzadeh AR, Omidi Y (2008) Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv 5:567–581
Zhang L, Li Y, Zhang C, Wang Y, Song C (2009) Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomed 4:175
Zawilska JB, Wojcieszak J, Olejniczak AB (2013) Prodrugs: a challenge for the drug development. Pharmacol Rep 65:1–14
Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C (2017) Prodrug approach: an overview of recent cases. Eur J Med Chem 127:810–827
Caruthers SD, Wickline SA, Lanza GM (2007) Nanotechnological applications in medicine. Curr Opin Biotechnol 18:26–30
Bisceglie V (1934) Über die antineoplastische Immunität. Z Krebsforsch 40:141–158
Nakama H, Ohsugi K, Otsuki T, Date I, Kosuga M, Okuyama T, Sakuragawa N (2006) Encapsulation cell therapy for mucopolysaccharidosis type VII using genetically engineered immortalized human amniotic epithelial cells. Tohoku J Exp Med 209:23–32
Baldo G, Mayer FQ, Burin M, Carrillo-Farga J, Matte U, Giugliani R (2012) Recombinant encapsulated cells overexpressing alpha-l-iduronidase correct enzyme deficiency in human mucopolysaccharidosis type I cells. Cells Tissues Organs 195:323–329
Baldo G, Mayer FQ, Martinelli B, Meyer FS, Burin M, Meurer L, Tavares AMV, Giugliani R, Matte U (2012) Intraperitoneal implant of recombinant encapsulated cells overexpressing alpha-l-iduronidase partially corrects visceral pathology in mucopolysaccharidosis type I mice. Cytotherapy 14:860–867
Diel D, Lagranha VL, Schuh RS, Bruxel F, Matte U, Teixeira HF (2018) Optimization of alginate microcapsules containing cells overexpressing α-l-iduronidase using Box–Behnken design. Eur J Pharm Sci 111:29–37
Omidi Y, Barar J (2012) Blood–brain barrier and effectiveness of therapy against brain tumors. In: Farassati F (ed) Novel therapeutic concepts in targeting glioma. IntechOpen Limited, London, pp 111–140
Pardridge WM (2010) Biopharmaceutical drug targeting to the brain. J Drug Target 18:157–167
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7:41–53
Omidi Y, Barar J (2012) Impacts of blood–brain barrier in drug delivery and targeting of brain tumors. BioImpacts 2:5
Sonoda H, Morimoto H, Yoden E, Koshimura Y, Kinoshita M, Golovina G, Takagi H, Yamamoto R, Minami K, Mizoguchi A (2018) A blood–brain-barrier-penetrating anti-human transferrin receptor antibody fusion protein for neuronopathic mucopolysaccharidosis II. Mol Ther 26:1366–1374
Fang F, Zou D, Wang W, Yin Y, Yin T, Hao S, Wang B, Wang G, Wang Y (2017) Non-invasive approaches for drug delivery to the brain based on the receptor mediated transport. Mater Sci Eng C 76:1316–1327
Giugliani R (2012) Mucopolysacccharidoses: from understanding to treatment, a century of discoveries. Genet Mol Biol 35:924–931
Mader KM, Beard H, King BM, Hopwood JJ (2008) Effect of high dose, repeated intra-cerebrospinal fluid injection of sulphamidase on neuropathology in mucopolysaccharidosis type IIIA mice. Genes Brain Behav 7:740–753
Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E (2007) Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab 91:61–68
Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M (2004) Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab 83:163–174
Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, Chen AH, Ponder KP, Haskins ME, Tippin BL, Le SQ, Passage MB, Guerra C, Dierenfeld A, Jens J, Snella E, Kan SH, Ellinwood NM (2010) Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol Genet Metab 101:115–122
Rattazzi M, Lanse S, McCullough R, Nester J, Jacobs E (1980) Towards enzyme replacement in GM2 gangliosidosis: organ disposition and induced central nervous system uptake of human beta-hexosaminidase in the cat. Birth Defects Orig Artic Ser 16:179–193
Boado RJ, Lu JZ, Hui EKW, Sumbria RK, Pardridge WM (2013) Pharmacokinetics and brain uptake in the rhesus monkey of a fusion protein of arylsulfatase a and a monoclonal antibody against the human insulin receptor. Biotechnol Bioeng 110:1456–1465
Mühlstein A, Gelperina S, Kreuter J (2013) Development of nanoparticle-bound arylsulfatase B for enzyme replacement therapy of mucopolysaccharidosis VI. Pharmazie 68:549–554
Donida B, Tauffner B, Raabe M, Immich MF, de Farias MA, de Sá Coutinho D, Machado AZ, Kessler RG, Portugal RV, Bernardi A (2018) Monoolein-based nanoparticles for drug delivery to the central nervous system: a platform for lysosomal storage disorder treatment. Eur J Pharm Biopharm 133:96–103
Acosta W, Ayala J, Dolan MC, Cramer CL (2015) RTB lectin: a novel receptor-independent delivery system for lysosomal enzyme replacement therapies. Sci Rep 5:14144
Poswar F, Baldo G, Giugliani R (2017) Phase I and II clinical trials for the mucopolysaccharidoses. Expert Opin Investig Drugs 26:1331–1340
Giugliani R, Nestrasil I, Chen S, Pardridge W, Rioux P (2017) Intravenous infusion of iduronidase-IgG and its impact on the central nervous system in children with Hurler syndrome. Mol Genet Metab 120:S55–S56
Pardridge WM, Boado RJ, Giugliani R, Schmidt M (2018) Plasma pharmacokinetics of valanafusp alpha, a human insulin receptor antibody-iduronidase fusion protein, in patients with mucopolysaccharidosis type I. BioDrugs 32:169–176
Boado RJ, Hui EK-W, Lu JZ, Sumbria RK, Pardridge WM (2013) Blood–brain barrier molecular trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey. Bioconjug Chem 24:1741–1749
Okuyama T, Sakai N, Yamamoto T, Yamaoka M, Tomio T (2018) Novel blood–brain barrier delivery system to treat CNS in MPS II: first clinical trial of anti-transferrin receptor antibody fused enzyme therapy. Mol Genet Metab 123:S109
Sohn YB, Cho SY, Lee J, Kwun Y, Huh R, Jin D-K (2015) Safety and efficacy of enzyme replacement therapy with idursulfase beta in children aged younger than 6 years with Hunter syndrome. Mol Genet Metab 114:156–160
Muenzer J, Gucsavas-Calikoglu M, McCandless SE, Schuetz TJ, Kimura A (2007) A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome). Mol Genet Metab 90:329–337
Sohn YB, Ko A-R, Seong M-R, Lee S, Kim MR, Cho SY, Kim J-S, Sakaguchi M, Nakazawa T, Kosuga M (2018) The efficacy of intracerebroventricular idursulfase-beta enzyme replacement therapy in mucopolysaccharidosis II murine model: heparan sulfate in cerebrospinal fluid as a clinical biomarker of neuropathology. J Inherit Metab Dis 41:1235–1246
Ghosh A, Shapiro E, Rust S, Delaney K, Parker S, Shaywitz AJ, Morte A, Bubb G, Cleary M, Bo T (2017) Recommendations on clinical trial design for treatment of mucopolysaccharidosis type III. Orphanet J Rare Dis 12:117
Rossomando A, Chen LL, Ciatto C, Liu J, Hu W, Hayes M, Rojas-Caro S, Quinn AG (2014) SBC-103, a recombinant human alpha-N-acetylglucosaminidase, demonstrates mannose-6-phosphate receptor dependent transport in an in vitro blood–brain barrier model. Mol Genet Metab 111:S91
Shaywitz AJ, Oh M, Kent S (2016) Design and rationale of the study programs for BMN 250, a novel enzyme replacement therapy (ERT) for Sanfilippo syndrome type B. Mol Genet Metab 117:S105–S106
Giugliani R, Dalla Corte A, Poswar F, Vanzella C, Horovitz D, Riegel M, Baldo G, Vairo F (2018) Intrathecal/intracerebroventricular enzyme replacement therapy for the mucopolysaccharidoses: efficacy, safety, and prospects. Expert Opin Orphan Drugs 6:403–411
Pintos-Morell G, Blasco-Alonso J, Couce ML, Gutiérrez-Solana LG, Guillén-Navarro E, O’Callaghan M, del Toro M (2018) Elosulfase alfa for mucopolysaccharidosis type IVA: real-world experience in 7 patients from the Spanish Morquio-A early access program. Mol Genet Metab Rep 15:116–120
Solanki GA, Sun PP, Martin KW, Hendriksz CJ, Lampe C, Guffon N, Hung A, Sisic Z, Shediac R, Harmatz PR (2016) Cervical cord compression in mucopolysaccharidosis VI (MPS VI): findings from the MPS VI Clinical Surveillance Program (CSP). Mol Genet Metab 118:310–318
Qi Y, McKeever K, Taylor J, Haller C, Song W, Jones SA, Shi J (2018) Pharmacokinetic and pharmacodynamic modeling to optimize the dose of vestronidase alfa, an enzyme replacement therapy for treatment of patients with mucopolysaccharidosis type VII: results from three trials. Clin Pharmacokinet 58:1–11
Harmatz P, Whitley CB, Wang RY, Bauer M, Song W, Jacobs K, Schwartz E, Haller C, Kakkis E (2017) A novel, randomized, placebo-controlled, blind-start, single-crossover phase 3 study to assess the efficacy and safety of UX003 (rhGUS) enzyme replacement therapy in patients with MPS VII. Mol Genet Metab 120:S63
Acknowledgements
Authors would like to acknowledge the Research Center for Pharmaceutical Nanotechnology and Connective Tissue Diseases Research Center at Tabriz University of Medical Sciences for financial support. The kind help from Dr. S. Sabermoghaddam and Mrs. R. Mousavi are highly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest to be disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safary, A., Akbarzadeh Khiavi, M., Omidi, Y. et al. Targeted enzyme delivery systems in lysosomal disorders: an innovative form of therapy for mucopolysaccharidosis. Cell. Mol. Life Sci. 76, 3363–3381 (2019). https://doi.org/10.1007/s00018-019-03135-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03135-z