Abstract
Seven transmembrane G protein-coupled receptors (GPCRs) have gained much interest in recent years as it is the largest class among cell surface receptors. G proteins lie in the heart of GPCRs signalling and therefore can be therapeutically targeted to overcome complexities in GPCR responses and signalling. G proteins are classified into four families (Gi, Gs, G12/13 and Gq); Gq is further subdivided into four classes. Among them Gαq and Gαq/11 isoforms are most crucial and ubiquitously expressed; these isoforms are almost 88% similar at their amino acid sequence but may exhibit functional divergences. However, uncertainties often arise about Gαq and Gαq/11 inhibitors, these G proteins might also have suitability to the invention of novel-specific inhibitors for each isoforms. YM-254890 and UBO-QIC are discovered as potent inhibitors of Gαq functions and also investigated in thrombin protease-activated receptor (PAR)-1 inhibitors and platelet aggregation inhibition. The most likely G protein involved in PAR-1 stimulates responses is one of the Gαq family isoforms. In this review, we highlight the molecular structures and pharmacological responses of Gαq family which may reflect the biochemical and molecular role of Gαq and Gαq/11. The advanced understanding of Gαq and Gαq/11 role in GPCR signalling may shed light on our understanding on cell biology, cellular physiology and pathophysiology and also lead to the development of novel therapeutic agents for a number of diseases.





Similar content being viewed by others
References
Lefkowitz RJ (2007) Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf). 190(1):9–19
Lodowski DT, Angel TE, Palczewski K (2009) Comparative analysis of GPCR crystal structures. Photochem Photobiol 85(2):425–430
Crouch MF, Osmond RI (2008) New strategies in drug discovery for GPCRs: high throughput detection of cellular ERK phosphorylation. Comb Chem High Throughput Screen. 11(5):344–356
Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, et al (2015) The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell Mol Life Sci 72(4):799–808
Zhao P, Metcalf M, Bunnett NW (2014) Biased signaling of protease-activated receptors. Front Endocrinol 5:67
Dulon S, Cande C, Bunnett NW, Hollenberg MD, Chignard M, Pidard D (2003) Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am J Respir Cell Mol Biol 28(3):339–346
Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W et al (2012) G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol 44(5):722–727
Little PJ (2013) GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Galphaq protein signalling pathways. Life Sci 92(20–21):951–956
Little PJ, Hollenberg MD, Kamato D, Thomas W, Chen J, Wang T, et al (2016) Integrating the GPCR transactivation-dependent and biased signalling paradigms in the context of PAR-1 signalling. Br J Pharmacol 173(20):2992–3000
Christopoulos A (2014) Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol 86(5):463–478
Jacobson KA (2015) New paradigms in GPCR drug discovery. Biochem Pharmacol 98(4):541–555
Daaka Y, Luttrell LM, Lefkowitz RJ (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390(6655):88–91
Krumins AM, Gilman AG (2006) Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem 281(15):10250–10262
Rodbell M (1992) The role of GTP-binding proteins in signal transduction: from the sublimely simple to the conceptually complex. Curr Top Cell Regul 32:1–47
Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H (2000) Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res 7(2):111–120
Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296(5573):1636–1639
Strathmann M, Simon MI (1990) G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci USA 87(23):9113–9117
Wilkie TM, Scherle PA, Strathmann MP, Slepak VZ, Simon MI (1991) Characterization of G-protein alpha subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc Natl Acad Sci USA. 88(22):10049–10053
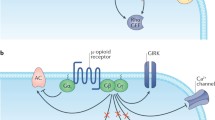
Mizuno N, Itoh H (2009) Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals 17(1):42–54
Kostenis E, Waelbroeck M, Milligan G (2005) Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci 26(11):595–602
Schrage R, Schmitz AL, Gaffal E, Annala S, Kehraus S, Wenzel D et al (2015) The experimental power of FR900359 to study Gq-regulated biological processes. Nat Commun. 6:10156
Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118(Pt 16):3573–3584
Zhang YE (2009) Non-Smad pathways in TGF-beta signaling. Cell Res 19(1):128–139
Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S et al (2013) Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal 25(10):2017–2024
Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H et al (2015) Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2:14
Pahlavan S, Oberhofer M, Sauer B, Ruppenthal S, Tian Q, Scholz A et al (2012) Galphaq and Galpha11 contribute to the maintenance of cellular electrophysiology and Ca2+ handling in ventricular cardiomyocytes. Cardiovasc Res 95(1):48–58
Rensing DT, Uppal S, Blumer KJ, Moeller KD (2015) Toward the selective inhibition of G proteins: total synthesis of a simplified YM-254890 analog. Org Lett 17(9):2270–2273
Kawasaki T, Taniguchi M, Moritani Y, Hayashi K, Saito T, Takasaki J et al (2003) Antithrombotic and thrombolytic efficacy of YM-254890, a G q/11 inhibitor, in a rat model of arterial thrombosis. Thromb Haemost 90(3):406–413
Taniguchi M, Nagai K, Arao N, Kawasaki T, Saito T, Moritani Y et al (2003) YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J Antibiot (Tokyo) 56(4):358–363
Grace MS, Lieu T, Darby B, Abogadie FC, Veldhuis N, Bunnett NW et al (2014) The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br J Pharmacol 171(16):3881–3894
Kaur H, Harris PW, Little PJ, Brimble MA (2015) Total synthesis of the cyclic depsipeptide YM-280193, a platelet aggregation inhibitor. Org Lett 17(3):492–495
Burch ML, Ballinger ML, Yang SN, Getachew R, Itman C, Loveland K et al (2010) Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. J Biol Chem 285(35):26798–26805
Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ (2013) Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem 288(10):7410–7419
Little PJ, Burch ML, Getachew R, Al-aryahi S, Osman N (2010) Endothelin-1 stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by endothelin receptor transactivation of the transforming growth factor-[beta] type I receptor. J Cardiovasc Pharmacol 56(4):360–368
Survase S, Ivey ME, Nigro J, Osman N, Little PJ (2005) Actions of calcium channel blockers on vascular proteoglycan synthesis: relationship to atherosclerosis. Vasc Health Risk Manag 1(3):199–208
Hall A (1990) The cellular functions of small GTP-binding proteins. Science 249(4969):635–640
Hein P, Bunemann M (2009) Coupling mode of receptors and G proteins. Naunyn Schmiedebergs Arch Pharmacol. 379(5):435–443
Hendriks-Balk MC, Peters SL, Michel MC, Alewijnse AE (2008) Regulation of G protein-coupled receptor signalling: focus on the cardiovascular system and regulator of G protein signalling proteins. Eur J Pharmacol 585(2–3):278–291
Hubbard KB, Hepler JR (2006) Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18(2):135–150
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411(6835):355–365
Lefkowitz RJ, Caron MG, Michel T, Stadel JM (1982) Mechanisms of hormone receptor-effector coupling: the beta-adrenergic receptor and adenylate cyclase. Fed Proc. 41(10):2664–2670
Liu X, Yu X, Zack DJ, Zhu H, Qian J (2008) TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinform 9:271
Yang X, Ye Y, Wang G, Huang H, Yu D, Liang S (2011) VeryGene: linking tissue-specific genes to diseases, drugs, and beyond for knowledge discovery. Physiol Genom 43(8):457–460
Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W et al (2016) Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal 28(1):110–119
Little PJ, Chait A, Bobik A (2011) Cellular and cytokine-based inflammatory processes as novel therapeutic targets for the prevention and treatment of atherosclerosis. Pharmacol Ther 131(3):255–268
Little PJ, Osman N, O’Brien KD (2008) Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol 19:448–454
Nigro J, Osman N, Dart AM, Little PJ (2006) Insulin resistance and atherosclerosis. Endocr Rev 27(3):242–259
Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ (2010) TGF-b stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad 2. Cell Mol Life Sci 67:2077–2090
Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A et al (2006) A signaling mechanism from G alpha q-protein-coupled metabotropic glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J Neurosci 26(3):971–980
Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM et al (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 100(19):10782–10787
Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S et al (2006) beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281(2):1261–1273
Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ (2005) beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem 280(9):8041–8050
Ahn S, Shenoy SK, Wei H, Lefkowitz RJ (2004) Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279(34):35518–35525
Miyamae S (1989) Influence of magnesium and extracellular calcium reduction on ouabain-treated sinoatrial node cells in rabbit heart. Pharmacol Toxicol 65(3):192–197
Fujioka M, Koda S, Morimoto Y, Biemann K (1988) Structure of Fr900359, a cyclic depsipeptide from Ardisia-Crenata sims. J Org Chem 53(12):2820–2825
Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S et al (2008) G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14(1):64–68
Zaima K, Deguchi J, Matsuno Y, Kaneda T, Hirasawa Y, Morita H (2013) Vasorelaxant effect of FR900359 from Ardisia crenata on rat aortic artery. J Nat Med 67(1):196–201
Kukkonen JP (2016) G-protein inhibition profile of the reported Gq/11 inhibitor UBO-QIC. Biochem Biophys Res Commun. 469(1):101–107
Carr R 3rd, Koziol-White C, Zhang J, Lam H, An SS, Tall GG et al (2016) Interdicting Gq activation in airway disease by receptor-dependent and receptor-independent mechanisms. Mol Pharmacol 89(1):94–104
Inamdar V, Patel A, Manne BK, Dangelmaier C, Kunapuli SP (2015) Characterization of UBO-QIC as a Galphaq inhibitor in platelets. Platelets 26(8):771–778
Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ (2014) Differential effects of the Gbeta5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol Pharmacol 85(5):758–768
Gao ZG, Jacobson KA (2016) On the selectivity of the Galphaq inhibitor UBO-QIC: a comparison with the Galphai inhibitor pertussis toxin. Biochem Pharmacol 107:59–66
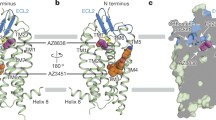
Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K et al (2004) A novel Galphaq/11-selective inhibitor. J Biol Chem 279(46):47438–47445
Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K et al (2010) Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci USA. 107(31):13666–13671
Kitatsuji C, Kurogochi M, Nishimura S, Ishimori K, Wakasugi K (2007) Molecular basis of guanine nucleotide dissociation inhibitor activity of human neuroglobin by chemical cross-linking and mass spectrometry. J Mol Biol 368(1):150–160
Tanski WJ, Roztocil E, Hemady EA, Williams JA, Davies MG (2004) Role of Galphaq in smooth muscle cell proliferation. J Vasc Surg 39(3):639–644
Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I et al (2006) Tricyclic pyrazoles. 4. Synthesis and biological evaluation of analogues of the robust and selective CB2 cannabinoid ligand 1-(2′,4′-dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c]pyrazo le-3-carboxamide. J Med Chem 49(25):7502–7512
Akhtar S, Yousif MH, Chandrasekhar B, Benter IF (2012) Activation of EGFR/ERBB2 via pathways involving ERK1/2, P38 MAPK, AKT and FOXO enhances recovery of diabetic hearts from ischemia-reperfusion injury. PLoS ONE 7(6):e39066
Pieper U, Webb BM, Dong GQ, Schneidman-Duhovny D, Fan H, Kim SJ et al (2014) ModBase, a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res. 42:D336–D346
Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R et al (2005) Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21(5):650–659
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamato, D., Mitra, P., Davis, F. et al. Gaq proteins: molecular pharmacology and therapeutic potential. Cell. Mol. Life Sci. 74, 1379–1390 (2017). https://doi.org/10.1007/s00018-016-2405-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2405-9