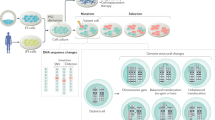
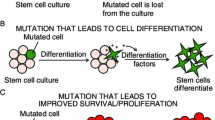
Abstract
Pluripotent stem cells differentiate into almost any specialized adult cell type of an organism. PSCs can be derived either from the inner cell mass of a blastocyst—giving rise to embryonic stem cells—or after reprogramming of somatic terminally differentiated cells to obtain ES-like cells, named induced pluripotent stem cells. The potential use of these cells in the clinic, for investigating in vitro early embryonic development or for screening the effects of new drugs or xenobiotics, depends on capability to maintain their genome integrity during prolonged culture and differentiation. Both human and mouse PSCs are prone to genomic and (epi)genetic instability during in vitro culture, a feature that seriously limits their real potential use. Culture-induced variations of specific chromosomes or genes, are almost all unpredictable and, as a whole, differ among independent cell lines. They may arise at different culture passages, suggesting the absence of a safe passage number maintaining genome integrity and rendering the control of genomic stability mandatory since the very early culture passages. The present review highlights the urgency for further studies on the mechanisms involved in determining (epi)genetic and chromosome instability, exploiting the knowledge acquired earlier on other cell types.

Similar content being viewed by others
References
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448:318–324
González F, Boué S, Izpisúa Belmonte JC (2011) Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet 12:231–242. doi:10.1038/nrg2937
Zapata AG, Alfaro D, García-Ceca J (2012) Biology of stem cells: the role of microenvironments. Adv Exp Med Biol 741:135–151. doi:10.1007/978-1-4614-2098-9_10
Tichy ED, Stambrook PJ (2008) DNA repair in murine embryonic stem cells and differentiated cells. Exp Cell Res 314:1929–1936. doi:10.1016/j.yexcr.2008.02.007
Giachino C, Orlando L, Turinetto V (2013) Maintenance of genomic stability in mouse embryonic stem cells: relevance in aging and disease. Int J Mol Sci 14:2617–2636. doi:10.3390/ijms14022617
Peterson SE, Loring JF (2014) Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem 289:4578–4584. doi:10.1074/jbc.R113.516419
Oliveira PH, da Silva CL, Cabral JM (2014) Concise review: genomic instability in human stem cells: current status and future challenges. Stem Cells 32:2824–2832. doi:10.1002/stem.1796
Rebuzzini P, Redi CA, Zuccotti M, Garagna S (2011) Genome stability in embryonic stem cells. In: Embryonic stem cells– recent advantages in pluripotent stem cell-based regenerative medicine Edited by Craig S, Atwood, p 399. doi: 10.5772/15091
Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K (2008) Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA 105:2883–2888. doi:10.1073/pnas.0711983105
Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW (2004) Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22:53–54
Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW (2007) Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol 25:207–215
Amps K, Andrews PW, Anyfantis G et al (2011) Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Int Stem Cell Initiat Nat Biotechnol 29:1132–1144. doi:10.1038/nbt.2051
Ben-David U, Benvenisty N (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 11:268–277. doi:10.1038/nrc3034
Taapken SM, Nisler BS, Newton MA, Sampsell-Barron TL, Leonhard KA, McIntire EM, Montgomery KD (2011) Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat Biotechnol 29:313–314. doi:10.1038/nbt.1835
Martins-Taylor K, Nisler BS, Taapken SM, Compton T, Crandall L, Montgomery KD, Lalande M, Xu RH (2011) Recurrent copy number variations in human induced pluripotent stem cells. Nat Biotechnol 29:488–491. doi:10.1038/nbt.1890
Garitaonandia I, Amir H, Boscolo FS, Wambua GK, Schultheisz HL, Sabatini K, Morey R, Waltz S, Wang YC, Tran H, Leonardo TR, Nazor K, Slavin I, Lynch C, Li Y, Coleman R, Gallego Romero I, Altun G, Reynolds D, Dalton S, Parast M, Loring JF, Laurent LC (2015) Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS One 10:e0118307. doi:10.1371/journal.pone.0118307
Olariu V, Harrison NJ, Coca D, Gokhale PJ, Baker D, Billings S, Kadirkamanathan V, Andrews PW (2010) Modeling the evolution of culture-adapted human embryonic stem cells. Stem Cell Res 4:50–56. doi:10.1016/j.scr.2009.09.001
Longo L, Bygrave A, Grosveld FG, Pandolfi PP (1997) The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimaerism. Transgenic Res 6:321–328
Sugawara A, Goto K, Sotomaru Y, Sofuni T, Ito T (2006) Current status of chromosomal abnormalities in mouse embryonic stem cell lines used in Japan. Comp Med 56:31–34
Kim YM, Lee JY, Xia L, Mulvihill JJ, Li S (2013) Trisomy 8: a common finding in mouse embryonic stem (ES) cell lines. Mol Cytogenet 6:3. doi:10.1186/1755-8166-6-3
Rebuzzini P, Neri T, Mazzini G, Zuccotti M, Redi CA, Garagna S (2008) Karyotype analysis of the euploid cell population of a mouse embryonic stem cell line revealed a high incidence of chromosome abnormalities that varied during culture. Cytogenet Genome Res 121:18–24. doi:10.1159/000124377
Rebuzzini P, Neri T, Zuccotti M, Redi CA, Garagna S (2008) Chromosome number variation in three mouse embryonic stem cell lines during culture. Cytotechnology 58:17–23. doi:10.1007/s10616-008-9164-x
Rebuzzini P, Pignalosa D, Mazzini G, Di Liberto R, Coppola A, Terranova N, Magni P, Redi CA, Zuccotti M, Garagna S (2012) Mouse embryonic stem cells that survive γ-rays exposure maintain pluripotent differentiation potential and genome stability. J Cell Physiol 227:1242–1249. doi:10.1002/jcp.22908
Ben-David U, Benvenisty N (2012) High prevalence of evolutionarily conserved and species-specific genomic aberrations in mouse pluripotent stem cells. Stem Cells 30:612–622. doi:10.1002/stem.1057
Gaztelumendi N, Nogués C (2014) Chromosome instability in mouse embryonic stem cells. Sci Rep 4:5324. doi:10.1038/srep05324
Rebuzzini P, Zuccotti M, Redi CA, Garagna S (2015) Chromosomal abnormalities in embryonic and somatic stem cells. Cytogenet Genome Res 147:1–9. doi:10.1159/000441645
Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N (2010) Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 7:521–531. doi:10.1016/j.stem.2010.07.017
Chen Q, Shi X, Rudolph C, Yu Y, Zhang D, Zhao X, Mai S, Wang G, Schlegelberger B, Shi Q (2011) Recurrent trisomy and Robertsonian translocation of chromosome 14 in murine iPS cell lines. Chromosome Res 19:857–868. doi:10.1007/s10577-011-9239-y
Pasi CE, Dereli-Öz A, Negrini S, Friedli M, Fragola G, Lombardo A, Van Houwe G, Naldini L, Casola S, Testa G, Trono D, Pelicci PG, Halazonetis TD (2011) Genomic instability in induced stem cells. Cell Death Differ 18:745–753. doi:10.1038/cdd.2011.9
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, Edel M, Boué S, Izpisúa Belmonte JC (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26:1276–1284. doi:10.1038/nbt.1503
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5:111–123. doi:10.1016/j.stem.2009.06.008
Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, MacDermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K (2011) A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol 29:279–286. doi:10.1038/nbt.1783
Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K (2011) Somatic coding mutations in human induced pluripotent stem cells. Nature 471:63–67. doi:10.1038/nature09805
Liu P, Kaplan A, Yuan B, Hanna JH, Lupski JR, Reiner O (2014) Passage number is a major contributor to genomic structural variations in mouse iPSCs. Stem Cells 32:2657–2667. doi:10.1002/stem.1779
Thompson SL, Bakhoum SF, Compton DA (2010) Mechanisms of chromosomal instability. Curr Biol 20:R285–R295
Holland AJ, Cleveland DW (2012) Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep 13:501–514. doi:10.1038/embor.2012.55
Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE (2007) Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol Biol Cell 18:3264–3276
Holubcová Z, Matula P, Sedláčková M, Vinarský V, Doležalová D, Bárta T, Dvořák P, Hampl A (2011) Human embryonic stem cells suffer from centrosomal amplification. Stem Cells 29:46–56. doi:10.1002/stem.549
Brevini TA, Pennarossa G, Maffei S, Tettamanti G, Vanelli A, Isaac S, Eden A, Ledda S, de Eguileor M, Gandolfi F (2012) Centrosome amplification and chromosomal instability in human and animal parthenogenetic cell lines. Stem Cell Rev 8:1076–1087. doi:10.1007/s12015-012-9379-2
Närvä E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P, Hovatta O, Otonkoski T, Tuuri T, Cui W, Brüstle O, Baker D, Maltby E, Moore HD, Benvenisty N, Andrews PW, Yli-Harja O, Lahesmaa R (2010) High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol 28:371–377. doi:10.1038/nbt.1615
Azuhata T, Scott D, Takamizawa S, Wen J, Davidoff A, Fukuzawa M, Sandler A (2001) The inhibitor of apoptosis protein survivin is associated with high-risk behavior of neuroblastoma. J Pediatr Surg 36:1785–1791
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 101:2999–3004
Caldas C, Brenton JD (2005) Sizing up miRNAs as cancer genes. Nat Med 11:712–714
Tanner MM, Tirkkonen M, Kallioniemi A, Isola J, Kuukasjärvi T, Collins C, Kowbel D, Guan XY, Trent J, Gray JW, Meltzer P, Kallioniemi OP (1996) Independent amplification and frequent co-amplification of three nonsyntenic regions on the long arm of chromosome 20 in human breast cancer. Cancer Res 56:3441–3445
Midorikawa Y, Yamamoto S, Ishikawa S, Kamimura N, Igarashi H, Sugimura H, Makuuchi M, Aburatani H (2006) Molecular karyotyping of human hepatocellular carcinoma using single-nucleotide polymorphism arrays. Oncogene 25:5581–5590
Koynova DK, Jordanova ES, Milev AD, Dijkman R, Kirov KS, Toncheva DI, Gruis NA (2007) Gene-specific fluorescence in situ hybridization analysis on tissue microarray to refine the region of chromosome 20q amplification in melanoma. Melanoma Res 17:37–41
Nguyen HT, Geens M, Mertzanidou A, Jacobs K, Heirman C, Breckpot K, Spits C (2014) Gain of 20q11.21 in human embryonic stem cells improves cell survival by increased expression of Bcl-xL. Mol Hum Reprod 20:168–177. doi:10.1093/molehr/gat077
Liang G, Zhang Y (2013) Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res 23:49–69
Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, Ku S, Martynova M, Semechkin R, Galat V, Gottesfeld J, Izpisua Belmonte JC, Murry C, Keirstead HS, Park HS, Schmidt U, Laslett AL, Muller FJ, Nievergelt CM, Shamir R, Loring JF (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8:106–118. doi:10.1016/j.stem.2010.12.003
Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E, Ng S, Sourour M, Hämäläinen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brüstle O, Bazett-Jones DP, Alitalo K, Lahesmaa R, Nagy A, Otonkoski T (2011) Copy number variation and selection during reprogramming to pluripotency. Nature 471:58–62. doi:10.1038/nature09871
Quinlan AR, Boland MJ, Leibowitz ML, Shumilina S, Pehrson SM, Baldwin KK, Hall IM (2011) Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell 9:366–373. doi:10.1016/j.stem.2011.07.018
Abyzov A, Mariani J, Palejev D, Zhang Y, Haney MS, Tomasini L, Ferrandino AF, Rosenberg Belmaker LA, Szekely A, Wilson M, Kocabas A, Calixto NE, Grigorenko EL, Huttner A, Chawarska K, Weissman S, Urban AE, Gerstein M, Vaccarino FM (2012) Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492:438–442. doi:10.1038/nature11629
Chen Y, Guo L, Chen J, Zhao X, Zhou W, Zhang C, Wang J, Jin L, Pei D, Zhang F (2014) Genome-wide CNV analysis in mouse induced pluripotent stem cells reveals dosage effect of pluripotent factors on genome integrity. BMC Genom 15:79. doi:10.1186/1471-2164-15-79
Martins-Taylor K, Xu RH (2012) Concise review: genomic stability of human induced pluripotent stem cells. Stem Cells 30:22–27. doi:10.1002/stem.705
Adams BR, Golding SE, Rao RR, Valerie K (2010a) Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants. PLoS One 5:e10001. doi: 10.1371/journal.pone.0010001
Adams BR, Hawkins AJ, Povirk LF, Valerie K (2010) ATM-independent, high-fidelity nonhomologous end joining predominates in human embryonic stem cells. Aging (Albany NY) 2:582–596
Hastings PJ, Lupski JR, Rosenberg SM, Ira G (2009) Mechanisms of change in gene copy number. Nat Rev Genet 10:551–564. doi:10.1038/nrg2593
Bogomazova AN, Lagarkova MA, Tskhovrebova LV, Shutova MV, Kiselev SL (2011) Error-prone nonhomologous end joining repair operates in human pluripotent stem cells during late G2. Aging (Albany NY) 3:584–596
Rithidech K, Bond VP, Cronkite EP, Thompson MH, Bullis JE (1995) Hypermutability of mouse chromosome 2 during the development of x-ray-induced murine myeloid leukemia. Proc Natl Acad Sci USA 92:1152–1156
Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A (2005) Genomic alterations in cultured human embryonic stem cells. Nat Genet 37:1099–1103
Lefort N, Feyeux M, Bas C, Féraud O, Bennaceur-Griscelli A, Tachdjian G, Peschanski M, Perrier AL (2008) Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat Biotechnol 26:1364–1366. doi:10.1038/nbt.1509
Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K (2008) Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol 26:1361–1363. doi:10.1038/nbt.1510
Liang Q, Conte N, Skarnes WC, Bradley A (2008) Extensive genomic copy number variation in embryonic stem cells. Proc Natl Acad Sci USA 105:17453–17456. doi:10.1073/pnas.0805638105
Arlt MF, Rajendran S, Birkeland SR, Wilson TE, Glover TW (2012) De novo CNV formation in mouse embryonic stem cells occurs in the absence of Xrcc4-dependent nonhomologous end joining. PLoS Genet 8:e1002981. doi:10.1371/journal.pgen.1002981
Richards RI, Sutherland GR (1992) Dynamic mutations: a new class of mutations causing human disease. Cell 70:709–712
Umar A, Kunkel TA (1996) DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur J Biochem 238:297–307
Meissner A (2010) Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol 28:1079–1088. doi:10.1038/nbt.1684
Ferguson-Smith AC (2011) Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet 12:565–575
Bibikova M, Chudin E, Wu B, Zhou L, Garcia EW, Liu Y, Shin S, Plaia TW, Auerbach JM, Arking DE, Gonzalez R, Crook J, Davidson B, Schulz TC, Robins A, Khanna A, Sartipy P, Hyllner J, Vanguri P, Savant-Bhonsale S, Smith AK, Chakravarti A, Maitra A, Rao M, Barker DL, Loring JF, Fan JB (2006) Human embryonic stem cells have a unique epigenetic signature. Genome Res 16:1075–1083
Allegrucci C, Young LE (2007) Differences between human embryonic stem cell lines. Hum Reprod Update 13:103–120
Silva SS, Rowntree RK, Mekhoubad S, Lee JT (2008) X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci USA 105:4820–4825. doi:10.1073/pnas.0712136105
Nagy A, Gócza E, Diaz EM, Prideaux VR, Iványi E, Markkula M, Rossant J (1990) Embryonic stem cells alone are able to support fetal development in the mouse. Development 110:815–821
Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, Gnirke A, Eggan K, Meissner A (2011) Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144:439–452. doi:10.1016/j.cell.2010.12.032
Dean W, Bowden L, Aitchison A, Klose J, Moore T, Meneses JJ, Reik W, Feil R (1998) Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development 125:2273–2282
Lund RJ, Närvä E, Lahesmaa R (2012) Genetic and epigenetic stability of human pluripotent stem cells. Nat Rev Genet 13:732–744. doi:10.1038/nrg3271
Nguyen HT, Geens M, Spits C (2013) Genetic and epigenetic instability in human pluripotent stem cells. Hum Reprod Update 19:187–205. doi:10.1093/humupd/dms048
Rugg-Gunn PJ, Ferguson-Smith AC, Pedersen RA (2005) Human embryonic stem cells as a model for studying epigenetic regulation during early development. Cell Cycle 4:1323–1326
Rugg-Gunn PJ, Ferguson-Smith AC, Pedersen RA (2007) Status of genomic imprinting in human embryonic stem cells as revealed by a large cohort of independently derived and maintained lines. Hum Mol Genet 16:R243–R251
Pick M, Stelzer Y, Bar-Nur O, Mayshar Y, Eden A, Benvenisty N (2009) Clone- and gene-specific aberrations of parental imprinting in human induced pluripotent stem cells. Stem Cells 27:2686–2690. doi:10.1002/stem.205
Li SS, Yu SL, Singh S (2010) Epigenetic states and expression of imprinted genes in human embryonic stem cells. World J Stem Cells 2:97–102. doi:10.4252/wjsc.v2.i4.97
Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H, Akutsu H, Umezawa A (2011) DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet 7:e1002085. doi:10.1371/journal.pgen.1002085
Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Müller FJ, Wang YC, Boscolo FS, Fakunle E, Dumevska B, Lee S, Park HS, Olee T, D’Lima DD, Semechkin R, Parast MM, Galat V, Laslett AL, Schmidt U, Keirstead HS, Loring JF, Laurent LC (2012) Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 10:620–634. doi:10.1016/j.stem.2012.02.013
Horii T, Hatada I (2013) Epigenetic instability in embryonic stem cells in “pluripotent stem cells”, edited by Deepa Bhartiya and Nibedita Lenka. INTECH Open Access Publisher. doi:10.5772/54367
Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM 3rd, Biniszkiewicz D, Yanagimachi R, Jaenisch R (2001) Epigenetic instability in ES cells and cloned mice. Science 293:95–97
Piedrahita JA (2011) The role of imprinted genes in fetal growth abnormalities. Birth Defects Res A Clin Mol Teratol 91:682–692. doi:10.1002/bdra.20795
Ishida M, Moore GE (2013) The role of imprinted genes in humans. Mol Aspects Med 34:826–840. doi:10.1016/j.mam.2012.06.009
Kouzarides T (2007) SnapShot: histone-modifying enzymes. Cell 131:822
Ludwig G, Nejman D, Hecht M, Orlanski S, Abu-Remaileh M, Yanuka O, Sandler O, Marx A, Roberts D, Benvenisty N, Bergman Y, Mendelsohn M, Cedar H (2014) Aberrant DNA methylation in ES cells. PLoS ONE 9:e96090. doi:10.1371/journal.pone.0096090
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326
Planello AC, Ji J, Sharma V, Singhania R, Mbabaali F, Müller F, Alfaro JA, Bock C, De Carvalho DD, Batada NN (2014) Aberrant DNA methylation reprogramming during induced pluripotent stem cell generation is dependent on the choice of reprogramming factors. Cell Regen (Lond) 3:4. doi:10.1186/2045-9769-3-4
Pollex T, Heard E (2012) Recent advances in X-chromosome inactivation research. Curr Opin Cell Biol 24:825–832. doi:10.1016/j.ceb.2012.10.007
Shen Y, Matsuno Y, Fouse SD, Rao N, Root S, Xu R, Pellegrini M, Riggs AD, Fan G (2008) X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc Natl Acad Sci USA 105:4709–4714. doi:10.1073/pnas.0712018105
Dvash T, Lavon N, Fan G (2010) Variations of X chromosome inactivation occur in early passages of female human embryonic stem cells. PLoS ONE 5:e11330. doi:10.1371/journal.pone.0011330
Lucchesi JC, Kelly WG, Panning B (2005) Chromatin remodeling in dosage compensation. Annu Rev Genet 39:615–651
Liu W, Yin Y, Jiang Y, Kou C, Luo Y, Huang S, Zheng Y, Li S, Li Q, Guo L, Gao S, Sun X (2011) Genetic and epigenetic X-chromosome variations in a parthenogenetic human embryonic stem cell line. J Assist Reprod Genet 28:303–313. doi:10.1007/s10815-010-9517-1
Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, Santagata S, Powers D, Barrett CB, Young RA, Lee JT, Jaenisch R, Mitalipova M (2010) Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell 141:872–883. doi:10.1016/j.cell.2010.04.010
Diaz Perez SV, Kim R, Li Z, Marquez VE, Patel S, Plath K, Clark AT (2012) Derivation of new human embryonic stem cell lines reveals rapid epigenetic progression in vitro that can be prevented by chemical modification of chromatin. Hum Mol Genet 21:751–764. doi:10.1093/hmg/ddr506
Tchieu J, Kuoy E, Chin MH, Trinh H, Patterson M, Sherman SP, Aimiuwu O, Lindgren A, Hakimian S, Zack JA, Clark AT, Pyle AD, Lowry WE, Plath K (2010) Female human iPSCs retain an inactive X chromosome. Cell Stem Cell 7:329–342. doi:10.1016/j.stem.2010.06.024
Mekhoubad S, Bock C, de Boer AS, Kiskinis E, Meissner A, Eggan K (2012) Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 10:595–609. doi:10.1016/j.stem.2012.02.014
Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS (2009) Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4:472–476. doi:10.1016/j.stem.2009.05.005
Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143:527–539. doi:10.1016/j.cell.2010.10.016
Bermejo-Alvarez P, Ramos-Ibeas P, Gutierrez-Adan A (2012) Solving the “X” in embryos and stem cells. Stem Cells Dev 21:1215–1224. doi:10.1089/scd.2011.0685
Minkovsky A, Patel S, Plath K (2012) Concise review: pluripotency and the transcriptional inactivation of the female Mammalian X chromosome. Stem Cells 30:48–54. doi:10.1002/stem.755
Navarro P, Chambers I, Karwacki-Neisius V, Chureau C, Morey C, Rougeulle C, Avner P (2008) Molecular coupling of Xist regulation and pluripotency. Science 321:1693–1695. doi:10.1126/science.1160952
van Bemmel JG, Mira-Bontenbal H, Gribnau J (2015) Cis- and trans-regulation in X inactivation. Chromosoma. doi:10.1007/s00412-015-0525-x
Putiri EL, Robertson KD (2011) Epigenetic mechanisms and genome stability. Clin Epigenetics 2:299–314
Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li E, Ueda HR, Nakayama J, Okano M (2006) Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells 11:805–814
Kimura H, Tada M, Nakatsuji N, Tada T (2004) Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol 24:5710–5720
Meshorer E, Misteli T (2006) Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol 7:540–546
Lonergan T, Bavister B, Brenner C (2007) Mitochondria in stem cells. Mitochondrion 7:289–296
Van Haute L, Spits C, Geens M, Seneca S, Sermon K (2013) Human embryonic stem cells commonly display large mitochondrial DNA deletions. Nat Biotechnol 31:20–23. doi:10.1038/nbt.2473
Prigione A, Lichtner B, Kuhl H, Struys EA, Wamelink M, Lehrach H, Ralser M, Timmermann B, Adjaye J (2011) Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell-like metabolic reprogramming. Stem Cells 29:1338–1348. doi:10.1002/stem.683
Pitceathly RD, Rahman S, Hanna MG (2012) Single deletions in mitochondrial DNA-molecular mechanisms and disease phenotypes in clinical practice. Neuromuscul Disord 22:577–586. doi:10.1016/j.nmd.2012.03.009
Wallace DC, Chalkia D (2013) Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Med 3:a021220
Alexeyev MF (2009) Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J 276:5768–5787. doi:10.1111/j.1742-4658.2009.07269.x
Catalina P, Montes R, Ligero G, Sanchez L, de la Cueva T, Bueno C, Leone PE, Menendez P (2008) Human ESCs predisposition to karyotypic instability: is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer 7:76. doi:10.1186/1476-4598-7-76
Peterson SE, Westra JW, Rehen SK, Young H, Bushman DM, Paczkowski CM, Yung YC, Lynch CL, Tran HT, Nickey KS, Wang YC, Laurent LC, Loring JF, Carpenter MK, Chun J (2011) Normal human pluripotent stem cell lines exhibit pervasive mosaic aneuploidy. PLoS ONE 6:e23018. doi:10.1371/journal.pone.0023018
Biancotti JC, Narwani K, Buehler N, Mandefro B, Golan-Lev T, Yanuka O, Clark A, Hill D, Benvenisty N, Lavon N (2010) Human embryonic stem cells as models for aneuploid chromosomal syndromes. Stem Cells 28:1530–1540. doi:10.1002/stem.483
Rebuzzini P, Fassina L, Mulas F, Bellazzi R, Redi CA, Di Liberto R, Magenes G, Adjaye J, Zuccotti M, Garagna S (2013) Mouse embryonic stem cells irradiated with γ-rays differentiate into cardiomyocytes but with altered contractile properties. Mutat Res 756:37–45. doi:10.1016/j.mrgentox.2013.06.007
Luft S, Pignalosa D, Nasonova E, Arrizabalaga O, Helm A, Durante M, Ritter S (2014) Fate of D3 mouse embryonic stem cells exposed to X-rays or carbon ions. Mutat Res, Genet Toxicol Environ Mutagen 760:56–63. doi:10.1016/j.mrgentox.2013.12.004
Ben-David U, Arad G, Weissbein U, Mandefro B, Maimon A, Golan-Lev T, Narwani K, Clark AT, Andrews PW, Benvenisty N, Carlos Biancotti J (2014) Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat Commun 5:4825. doi:10.1038/ncomms5825
Bosman A, Letourneau A, Sartiani L, Del Lungo M, Ronzoni F, Kuziakiv R, Tohonen V, Zucchelli M, Santoni F, Guipponi M, Dumevska B, Hovatta O, Antonarakis SE, Jaconi ME (2015) Perturbations of heart development and function in cardiomyocytes from human embryonic stem cells with trisomy 21. Stem Cells 33:1434–1446. doi:10.1002/stem.1961
Wang CC, Kazuki Y, Oshimura M, Ikeo K, Gojobori T (2011) Gene dosage imbalance of human chromosome 21 in mouse embryonic stem cells differentiating to neurons. Gene 481:93–101. doi:10.1016/j.gene.2011.04.003
Ruiz S, Lopez-Contreras AJ, Gabut M, Marion RM, Gutierrez-Martinez P, Bua S, Ramirez O, Olalde I, Rodrigo-Perez S, Li H, Marques-Bonet T, Serrano M, Blasco MA, Batada NN, Fernandez-Capetillo O (2015) Limiting replication stress during somatic cell reprogramming reduces genomic instability in induced pluripotent stem cells. Nat Commun 6:8036. doi:10.1038/ncomms9036
Gao Y, Han Z, Li Q, Wu Y, Shi X, Ai Z, Du J, Li W, Guo Z, Zhang Y (2015) Vitamin C induces a pluripotent state in mouse embryonic stem cells by modulating microRNA expression. FEBS J 282:685–699. doi:10.1111/febs.13173
Otsuji TG, Bin J, Yoshimura A, Tomura M, Tateyama D, Minami I, Yoshikawa Y, Aiba K, Heuser JE, Nishino T, Hasegawa K, Nakatsuji N (2014) A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Reports 2:734–745. doi:10.1016/j.stemcr.2014.03.012
Lou YR, Kanninen L, Kuisma T, Niklander J, Noon LA, Burks D, Urtti A, Yliperttula M (2014) The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev 23:380–392. doi:10.1089/scd.2013.0314
Lei Y, Jeong D, Xiao J, Schaffer DV (2014) Developing defined and scalable 3D culture systems for culturing human pluripotent stem cells at high densities. Cell Mol Bioeng 7:172–183
Xu K, Narayanan K, Lee F, Bae KH, Gao S, Kurisawa M (2015) Enzyme-mediated hyaluronic acid-tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater 24:159–171. doi:10.1016/j.actbio.2015.06.026
Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, Mizuguchi H (2013) 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 34:1781–1789. doi:10.1016/j.biomaterials.2012.11.029
Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR (2015) Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61:1370–1381. doi:10.1002/hep.27621
Lew DJ, Burke DJ (2003) The spindle assembly and spindle position checkpoints. Annu Rev Genet 37:251–282
Musacchio A (2011) Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci 366:3595–3604. doi:10.1098/rstb.2011.0072
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8:379–393
Rao CV, Yamada HY, Yao Y, Dai W (2009) Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice. Carcinogenesis 30:1469–1474. doi:10.1093/carcin/bgp081
Thompson SL, Compton DA (2008) Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol 180:665–672
Bakhoum SF, Thompson SL, Manning AL, Compton DA (2009) Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol 11:27–35. doi:10.1038/ncb1809
Torres EM, Williams BR, Amon A (2008) Aneuploidy: cells losing their balance. Genetics 179:737–746. doi:10.1534/genetics.108.090878
Tanaka K, Hirota T (2009) Chromosome segregation machinery and cancer. Cancer Sci 100:1158–1165. doi:10.1111/j.1349-7006.2009.01178.x
Silkworth WT, Nardi IK, Paul R, Mogilner A, Cimini D (2012) Timing of centrosome separation is important for accurate chromosome segregation. Mol Biol Cell 23:401–411. doi:10.1091/mbc.E11-02-0095
Gregan J, Polakova S, Zhang L, Tolić-Nørrelykke IM, Cimini D (2011) Merotelic kinetochore attachment: causes and effects. Trends Cell Biol 21:374–381. doi:10.1016/j.tcb.2011.01.003
Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED (2001) Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol 153:517–527
Adams RR, Carmena M, Earnshaw WC (2001) Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol 11:49–54
Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ (2002) Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J Cell Biol 158:841–847
Guerrero AA, Martínez AC, van Wely KH (2010) Merotelic attachments and non-homologous end joining are the basis of chromosomal instability. Cell Div 5:13. doi:10.1186/1747-1028-5-13
Salisbury JL, Whitehead CM, Lingle WL, Barrett SL (1999) Centrosomes and cancer. Biol Cell 91:451–460
Krämer A, Neben K, Ho AD (2002) Centrosome replication, genomic instability and cancer. Leukemia 16:767–775
Strnad P, Gönczy P (2008) Mechanisms of procentriole formation. Trends Cell Biol 18:389–396. doi:10.1016/j.tcb.2008.06.004
Loncarek J, Khodjakov A (2009) Ab ovo or de novo? Mechanisms of centriole duplication. Mol Cells 27:135–142. doi:10.1007/s10059-009-0017-z
Nigg EA, Stearns T (2011) The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13:1154–1160. doi:10.1038/ncb2345
Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW (2010) Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol 188:191–198. doi:10.1083/jcb.200911102
Nigg EA (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol 10:776–783
McCoy RC, Demko Z, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Fraser HB, Petrov DA (2015) Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Science 348:235–238. doi:10.1126/science.aaa3337
Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91:35–45
Iwaizumi M, Shinmura K, Mori H, Yamada H, Suzuki M, Kitayama Y, Igarashi H, Nakamura T, Suzuki H, Watanabe Y, Hishida A, Ikuma M, Sugimura H (2009) Human Sgo1 downregulation leads to chromosomal instability in colorectal cancer. Gut 58:249–260. doi:10.1136/gut.2008.149468
Zhang N, Ge G, Meyer R, Sethi S, Basu D, Pradhan S, Zhao YJ, Li XN, Cai WW, El-Naggar AK, Baladandayuthapani V, Kittrell FS, Rao PH, Medina D, Pati D (2008) Overexpression of Separase induces aneuploidy and mammary tumorigenesis. Proc Natl Acad Sci USA 105:13033–13038. doi:10.1073/pnas.0801610105
Gu W, Zhang F, Lupski JR (2008) Mechanisms for human genomic rearrangements. Pathogenetics 1:4. doi:10.1186/1755-8417-1-4
Stankiewicz P, Lupski JR (2002) Genome architecture, rearrangements and genomic disorders. Trends Genet 18:74–82
Bailey JA, Eichler EE (2006) Primate segmental duplications: crucibles of evolution, diversity and disease. Nat Rev Genet 7:552–564
Arlt MF, Wilson TE, Glover TW (2012) Replication stress and mechanisms of CNV formation. Curr Opin Genet Dev 22:204–210. doi:10.1016/j.gde.2012.01.009
Lieber MR, Gu J, Lu H, Shimazaki N, Tsai AG (2010) Nonhomologous DNA end joining (NHEJ) and chromosomal translocations in humans. Subcell Biochem 50:279–296. doi:10.1007/978-90-481-3471-7_14
Woodward KJ, Cundall M, Sperle K, Sistermans EA, Ross M, Howell G, Gribble SM, Burford DC, Carter NP, Hobson DL, Garbern JY, Kamholz J, Heng H, Hodes ME, Malcolm S, Hobson GM (2005) Heterogeneous duplications in patients with Pelizaeus-Merzbacher disease suggest a mechanism of coupled homologous and nonhomologous recombination. Am J Hum Genet 77:966–987
Lee JH, Kim KH, Morio T, Kim H (2006) Ataxia-telangiectasia-mutated-dependent activation of Ku in human fibroblasts exposed to hydrogen peroxide. Ann N Y Acad Sci 1091:76–82
Lee JA, Carvalho CM, Lupski JR (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131:1235–1247
Wells RD (1996) Molecular basis of genetic instability of triplet repeats. J Biol Chem 271:2875–2878
Viguera E, Canceill D, Ehrlich SD (2001) Replication slippage involves DNA polymerase pausing and dissociation. EMBO J 20:2587–2595
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138(2073–2087):e3. doi:10.1053/j.gastro.2009.12.064
Bestor TH (1992) Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO J 11:2611–2617
Leonhardt H, Page AW, Weier HU, Bestor TH (1992) A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71:865–873
Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, Laird PW, Jones PA (2002) Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol 22:480–491. doi:10.1038/cr.2012.175
Yokochi T, Robertson KD (2002) Preferential methylation of unmethylated DNA by Mammalian de novo DNA methyltransferase Dnmt3a. J Biol Chem 277:11735–11745
Chedin F, Lieber MR, Hsieh CL (2002) The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc Natl Acad Sci USA 99:16916–16921
Suetake I, Shinozaki F, Miyagawa J, Takeshima H, Tajima S (2004) DNMT3L stimulates the DNA methylation activity of Dnmt3a and Dnmt3b through a direct interaction. J Biol Chem 279:27816–27823
Li JY, Pu MT, Hirasawa R, Li BZ, Huang YN, Zeng R, Jing NH, Chen T, Li E, Sasaki H, Xu GL (2007) Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol 27:8748–8759
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080
Yang RF, Li CM, Qiu HR, Lu H, Wu HX, Xu JR, Li JY, Chen LJ (2010) Investigation of chromosome 1 aberrations in patients with multiple myeloma using cIg-FISH method and its significance. Zhonghua Xue Ye Xue Za Zhi 31:804–808
Imreh MP, Gertow K, Cedervall J, Unger C, Holmberg K, Szöke K, Csöregh L, Fried G, Dilber S, Blennow E, Ahrlund-Richter L (2006) In vitro culture conditions favoring selection of chromosomal abnormalities in human ES cells. J Cell Biochem 99:508–516
Avery S, Hirst AJ, Baker D, Lim CY, Alagaratnam S, Skotheim RI, Lothe RA, Pera MF, Colman A, Robson P, Andrews PW, Knowles BB (2013) BCL-XL mediates the strong selective advantage of a 20q11.21 amplification commonly found in human embryonic stem cell cultures. Stem Cell Reports 1:379–386. doi:10.1016/j.stemcr.2013.10.005
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rebuzzini, P., Zuccotti, M., Redi, C.A. et al. Achilles’ heel of pluripotent stem cells: genetic, genomic and epigenetic variations during prolonged culture. Cell. Mol. Life Sci. 73, 2453–2466 (2016). https://doi.org/10.1007/s00018-016-2171-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2171-8