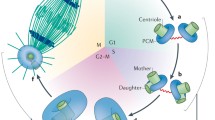
Abstract
The centrosome, an organelle comprising centrioles and associated pericentriolar material, is the major microtubule organizing center in animal cells. For the cell to form a bipolar mitotic spindle and ensure proper chromosome segregation at the end of each cell cycle, it is paramount that the cell contains two and only two centrosomes. Because the number of centrosomes in the cell is determined by the number of centrioles, cells have evolved elaborate mechanisms to control centriole biogenesis and to tightly coordinate this process with DNA replication. Here we review key proteins involved in centriole assembly, compare two major modes of centriole biogenesis, and discuss the mechanisms that ensure stringency of centriole number.
Similar content being viewed by others
References
Alvey, P.L. (1985). An investigation of the centriole cycle using 3T3 and CHO cells. J. Cell Sci. 78, 147–162.
Balczon, R., Bao, L., Zimmer, W.E., Brown, K., Zinkowski, R.P., and Brinkley, B.R. (1995). Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130, 105–115.
Berthet, C., Aleem, E., Coppola, V., Tessarollo, L., and Kaldis, P. (2003). Cdk2 Knockout mice are viable. Curr. Biol. 13, 1775–1785.
Bettencourt-Dias, M., and Carvalho-Santos, Z. (2008). Double life of centrioles: CP110 in the spotlight. Trends. Cell Biol. 18, 8–11.
Bettencourt-Dias, M., Rodrigues-Martins, A., Carpenter, L., Riparbelli, M., Lehmann, L, Gatt, M.K., Carmo, N., Balloux, F., Callaini, G., and Glover, D.M. (2005). Sak/Plk4 is required for centriole duplication and flagella development. Curr. Biol. 15, 2199–2207.
Bisgrove, B.W., and Yost, H.J. (2006). The roles of cilia in developmental disorders and disease. Development 133, 4131–4143.
Blow, J.J., and Dutta, A. (2005). Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell Biol. 6, 476–486.
Bobinnec, Y., Khodjakov, A, Mir, L.M., Rieder, C.L., Edde, B., and Bornens, M. (1998). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575–1589.
Chen, Z., Indjeian, V.B., McManus, M., Wang, L., and Dynlacht, B.D. (2002). CP110, a cell cycle-dependent Cdk substrate, regulates centrosome duplication in human cells. Dev. Cell 3, 339–350.
Chretien, D., Buendia, B., Fuller, S.D., and Karsenti, E. (1997). Reconstruction of the centrosome cycle from cryoelectron micrographs. J. Struct. Biol. 120, 117–133.
Dammermann, A., Muller-Reichert, T., Pelletier, L., Habermann, B., Desai, A., and Oegema, K. (2004). Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell 7, 815–829.
Dammermann, A., Maddox, P.S., Desai, A., and Oegema, K. (2008). SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the γ-tubulin-mediated addition of centriolar microtubules. J. Cell Biol. 180, 771–785.
Dawe, H.R., Farr, H., and Gull, K. (2007). Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci 120, 7–15.
Delattre, M., and Gonczy, P. (2004). The arithmetic of centrosome biogenesis. J. Cell Sci. 117, 1619–1630.
Delattre, M., Canard, C., and Gonczy, P. (2006). Sequential protein recruitment in C. elegans centriole formation. Curr. Biol. 16, 1844–1849.
Dippell, R. (1968). The development of basal bodies in Paramecium. Proc. Natl. Acad. Sci. USA 61, 461–468.
Dirksen, E.R. (1991). Centriole and basal body formation during ciliogenesis revisited. Biol. Cell 72, 31–38.
Dix, C.I., and Raff, J.W. (2007). Drosophila Spd-2 recruits PCM to the sperm centriole, but is dispensable for centriole duplication. Curr. Biol. 17, 1759–1764.
Duensing, S., and Munger, K. (2003). Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J. Virol. 77, 12331–12335.
Duensing, A., Liu, Y., Perdreau, S.A., Kleylein-Sohn, J., Nigg, E.A., and Duensing, S. (2007). Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene 26, 6280–6288.
Dutcher, S.K. (2007). Finding treasures in frozen cells: new centriole intermediates. Bioessays 29, 630–634.
Fisk, H.A., and Winey, M. (2001). The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106, 95–104.
Fisk, H.A, Mattison, O.P., and Winey, M. (2003). Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc. Natl. Acad. Sci. USA 100, 14875–14880.
Fuller, S.D., Gowen, B.E., Reinsch, S., Sawyer, A., Buendia, B., Wepf, R., and Karsenti, E. (1995). The core of the mammalian centriole contains γ-tubulin. Curr. Biol. 5, 1384–1393.
Geng, Y., Yu, Q., Sicinska, E., Das, M., Schneider, J.E., Bhattacharya, S., Rideout, W.M., Bronson, R.T., Gardner, H., and Sicinski, P. (2003). Cyclin E ablation in the mouse. Cell 114, 431–443.
Hinchcliffe, E.H., and Sluder, G. (2001a). It takes two to tango: understanding how centrosome duplication is regulated through-houtthe cell cycle. Genes Dev. 15, 1167–1181.
Hinchcliffe, E.H., and Sluder, G. (2001b). Centrosome duplication: Three kinases come up a winner! Curr. Biol. 11, R698–R701.
Hiraki, M., Nakazawa, Y., Kamiya, R., and Hirono, M. (2007). Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr. Biol. 17, 1778–1783.
Hook, S.S., Lin, J.J., and Dutta, A. (2007). Mechanisms to control re-replication and implications for cancer. Curr. Opin. Cell Biol. 19, 663–671.
Jones, M.H., and Winey, M. (2006). Centrosome duplication: is asymmetry the clue? Curr. Biol. 16, R808–810.
Kasbek, O., Yang, O.H., Yusof, A.M., Chapman, H.M., Winey, M., and Fisk, H.A. (2007). Preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Mol. Biol. Cell 18, 4457–4469.
Kemp, C.A., Kopish, K.R., Zipperlen, P., Ahringer, J., and O’Connell, K.F. (2004). Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev. Cell 6, 511–523.
Keryer, G., Ris, H., and Borisy, G.G. (1984). Centriole distribution during tripolar mitosis in Chinese hamster ovary cells. J. Cell Biol. 98, 2222–2229.
Khodjakov, A., Rieder, C.L., Sluder, G., Cassels, G., Sibon, O., and Wang, C.L. (2002). De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171–1181.
Kirkham, M., Muller-Reichert, T., Oegema, K., Grill, S., and Hyman, A. A. (2003). SAS-4 Is a C. elegans centriolar protein that controls centrosome size. Cell 112, 575–587.
Kleylein-Sohn, J., Westendorf, J., Le Clech, M., Habedanck, R., Stierhof, Y.D., and Nigg, E.A. (2007). Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202.
Kuriyama, R., and Borisy, G.G. (1981). Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J. Cell Biol. 91, 814–821.
Kuriyama, R., and Borisy, G.G. (1983). Cytasters induced within unfertilized sea-urchin eggs. J. Cell Sci. 61, 175–189.
La Terra, S., English, C.N., Hergert, P., McEwen, B.F., Sluder, G., and Khodjakov, A. (2005). The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J. Cell Biol. 168, 713–722.
Leidel, S., Delattre, M., Cerutti, L, Baumer, K., and Gonczy, P. (2005). SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7, 115–125.
Loncarek, J., Hergert, P., Magidson, V., and Khodjakov, A. (2008). Control of daughter centriole formation by the pericentriolar material. Nat. Cell Biol. 10, 322–328.
Mahowald, A.P., Caulton, J.H., Edwards, M.K., and Floyd, A.D. (1979). Loss of centrioles and polypioidization in follicle cells of Drosophila melanogaster. Exp. Cell Res. 118, 404–410.
Manandhar, G., Schatten, H., and Sutovsky, P. (2005). Centrosome reduction during gametogenesis and its significance. Biol. Reprod. 72, 2–13.
Marshall, W.F. (2007). Centriole assembly: the origin of nine-ness. Cur. Biol. 17, R1057–R1059.
Marshall, W.F. (2008). The cell biological basis of ciliary disease. J. Cell Biol. 180, 17–21.
Matsumoto, Y., and Maller, J.L. (2004). A centrosomal localization signal in cyclin E required for cdk2-independent S phase entry. Science 306, 885–888.
Matsumoto, Y., Hayashi, K., and Nishida, E. (1999). Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 9, 429–432.
Mazia D. (1987). The multiplicity of the mitotic centers and the time-course of their duplication and separation. Biophys. Biochem. Cytol. 7, 1–20.
Meraldi, P., Lukas, J., Fry, A.M., Bartek, J., and Nigg, E.A. (1999). Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 1, 88–93.
Moritz, M., Braunfeld, M.B., Guenebaut, V., Heuser, J., and Agard, D.A. (2000). Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365–370.
Moudjou, M., Bordes, N., Paintrand, M., and Bornens, M. (1996). γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109, 875–887.
Nakazawa, Y., Hiraki, M., Kamiya, R., and Hirono, M. (2007). SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 17, 2169–2174.
Nigg, E.A. (2007). Centriole duplication: of rules and licenses. Trends Cell Biol. 17, 215–221.
O’Connell, K.F., Caron, C., Kopish, K.R., Hurd, D.D., Kemphues, K.J., Li, Y., and White, J.G. (2001). The C. ielegans Zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105, 547–558.
O’Toole, E.T., Giddings, T.H., Mcintosh, J.R., and Dutcher, S.K. (2003). Three-dimensional organization of basal bodies from wild-type and δ-tubulin deletion strains of Chlamydomonas reinhardtii. Mol. Biol. Cell 14, 2999–3012.
Okuda, M., Horn, H.F., Tarapore, P., Tokuyama, Y., Smulian, A.G., Chan, P.K., Knudsen, E.S., Hofmann, I.A., Snyder, J.D., Bove, K.E., et al. (2000). Nucleophosmin/B23 is a target of CDK2/Cy-clin E in centrosome duplication. Cell 103, 127–140.
Peel, N., Stevens, N.R., Basto, R., and Raff, J.W. (2007). Overex-pressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 17, 834–843.
Pelletier, L., Ozlu, N., Hannak, E., Cowan, C., Habermann, B., Ruer, M., Muller-Reichert, T., and Hyman, A.A. (2004). The Caenor-habditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 14, 863–873.
Pelletier, L., Toole, E., Schwager, A, Hyman, A.A, and Muller-Reichert, T. (2006). Centriole assembly in Caenorhabditis elegans. Nature 444, 619–623.
Piel, M., Nordberg, J., Euteneuer, U., and Bornens, M. (2001). Cen-trosome-dependent exit of cytokinesis in animal cells. Science 291, 1550–1553
Riparbelli, M.G., and Callaini, G. (2003). Drosophila parthenogenesis: a model for de novo centrosome assembly. Dev. Biol. 260, 298–313.
Rodrigues-Martins, A., Riparbelli, M., Callaini, G., Glover, D.M., and Bettencourt-Dias, M. (2007a). Revisiting the role of the mother centriole in centriole biogenesis. Science 316, 1046–1050.
Rodrigues-Martins, A., Bettencourt-Dias, M.n., Riparbelli, M., Ferreira, C, Ferreira, I., Callaini, G., and Glover, D.M. (2007b). DSAS-6 Organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr. Biol. 17, 1465–1472.
Salisbury, J.L., Suino, K.M., Busby, R., and Springett, M. (2002). Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12, 1287–1292.
Silflow, C.D., Liu, B., LaVoie, M., Richardson, E.A., and Palevitz, B.A (1999). γ-Tubulin in Chlamydomonas: characterization of the gene and localization of the gene product in cells. Cell Motil. Cytoskeleton 42, 285–297.
Sluder, G., and Begg, D.A. (1985). Experimental analysis of the reproduction of spindle poles. J. Cell Sci. 76, 35–51.
Sorokin, S.P. (1968). Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230.
Spektor, A., Tsang, W.Y., Khoo, D., and Dynlacht, B.D. (2007). Cep97 and CP110 Suppress a cilia assembly program. Cell 130, 678–690.
Strnad, P., and Gönczy, P. (2008). Mechanisms of procentriole formation. Trends. Cell Biol. 18, 389–396.
Strnad, P., Leidel, S., Vinogradova, T., Euteneuer, U., Khodjakov, A., and Gonczy, P. (2007). Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell 13, 203–213.
Stucke, V.M., Sillje, H.H., Arnaud, L, and Nigg, E.A. (2002). Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21, 1723–1732.
Szollosy, D., Calarco, P., and Donahue, R.P. (1972). Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 11, 521–541.
Szöllosi, D., and Ozil, J.P. (1991). De novo formation of centrioles in parthenogenetically activated, diploidized rabbit embryos. Biol. Cell 72, 61–66.
Tokuyama, Y., Horn, H.F., Kawamura, K., Tarapore, P., and Fukasawa, K. (2001). Specific phosphorylation of nucleo-phosmin on Thr199 by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J. Biol. Chem. 276, 21529–21537.
Tsou, M.F.B., and Stearns, T. (2006a). Controlling centrosome number: licenses and blocks. Curr. Opin. Cell Biol. 18, 74–78.
Tsou, M.F.B., and Stearns, T. (2006b). Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947–951.
Uetake, Y., Loncarek, J., Nordberg, J.J., English, C.N., La Terra, S., Khodjakov, A., and Sluder, G. (2007). Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J. Cell Biol. 176, 173–182.
Vladar, E.K., and Stearns, T. (2007). Molecular characterization of centriole assembly in ciliated epithelial cells. J. Cell Biol. 178, 31–42.
Vorobjev, I.A., and Chentsov, Y. (1982). Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 93, 938–949.
Winey, M., Goetsch, L., Baum, P., and Byers, B. (1991). Mps1 and Mps2: novel yeast genes defining distinct steps of spindle pole body duplication. J. Cell Biol. 114, 745–754.
Winkles, J.A., and Alberts, G.F. (2005). Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene 24, 260–266.
Wong, C., and Stearns, T. (2003). Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 5, 539–544.
Young, A., Dictenberg, J.B., Purohit, A., Tuft, R., and Doxsey, S.J. (2000). Cytoplasmic dynein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol. Biol. Cell 11, 2047–2056.
Zhu, F., Lawo, S., Bird, A, Pinchev, D., Ralph, A., Richter, C, Muller-Reichert, T., Kittler, R., Hyman, A.A, and Pelletier, L (2008). The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr. Biol. 18, 136–141.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Loncarek, J., Khodjakov, A. Ab ovo or de novo? Mechanisms of centriole duplication. Mol Cells 27, 135–142 (2009). https://doi.org/10.1007/s10059-009-0017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-009-0017-z