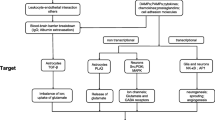
Abstract
Epilepsy is a group of chronic neurological disorders that have diverse etiologies but are commonly characterized by spontaneous seizures and behavioral comorbidities. Although the mechanisms underlying the epileptic seizures mostly remain poorly understood and the causes often can be idiopathic, a considerable portion of cases are known as acquired epilepsy. This form of epilepsy is typically associated with prior neurological insults, which lead to the initiation and progression of epileptogenesis, eventually resulting in unprovoked seizures. A convergence of evidence in the past two decades suggests that inflammation within the brain may be a major contributing factor to acquired epileptogenesis. As evidenced in mounting preclinical and human studies, neuroinflammatory processes, such as activation and proliferation of microglia and astrocytes, elevated production of pro-inflammatory cytokines and chemokines, blood–brain barrier breakdown, and upregulation of inflammatory signaling pathways, are commonly observed after seizure-precipitating events. An increased knowledge of these neuroinflammatory processes in the epileptic brain has led to a growing list of inflammatory mediators that can be leveraged as potential targets for new therapies of epilepsy and/or biomarkers that may provide valued information for the diagnosis and prognosis of the otherwise unpredictable seizures. In this review, we mainly focus on the most recent progress in understanding the roles of these inflammatory molecules in acquired epilepsy and highlight the emerging evidence supporting their candidacy as novel molecular targets for new pharmacotherapies of acquired epilepsy and the associated behavioral deficits.


Similar content being viewed by others
References
Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P. Epilepsy Nat Rev Dis Primers. 2018;4:18024. https://doi.org/10.1038/nrdp.2018.24.
Jiang J, Santhakumar V, Zhu X. Editorial: Neuroinflammation in acquired epilepsy. Frontiers in Cell and Developmental Biology 2022; https://doi.org/10.3389/fcell.2022.1074537.
Erisken S, Nune G, Chung H, Kang JW, Koh S. Time and age dependent regulation of neuroinflammation in a rat model of mesial temporal lobe epilepsy: Correlation with human data. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.969364.
Gage M, Gard M, Thippeswamy T. Characterization of cortical glial scars in the diisopropylfluorophosphate (DFP) rat model of epilepsy. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.867949.
Patel DC, Thompson EG, Sontheimer H. Brain-derived neurotrophic factor inhibits the function of cation-chloride cotransporter in a mouse model of viral infection-induced epilepsy. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.961292.
Zilberter Y, Popova I, Zilberter M. Unifying mechanism behind the onset of acquired epilepsy. Trends Pharmacol Sci. 2022;43:87–96. https://doi.org/10.1016/j.tips.2021.11.009.
McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15-22. https://doi.org/10.1038/399a015.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshe SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–21. https://doi.org/10.1111/epi.13709.
Alyu F, Dikmen M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr. 2017;29:1–16. https://doi.org/10.1017/neu.2016.47.
Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–72. https://doi.org/10.1038/s41582-019-0217-x.
Simonato M, Agoston DV, Brooks-Kayal A, Dulla C, Fureman B, Henshall DC, Pitkanen A, Theodore WH, Twyman RE, Kobeissy FH, Wang KK, Whittemore V, Wilcox KS. Identification of clinically relevant biomarkers of epileptogenesis - a strategic roadmap. Nat Rev Neurol. 2021;17:231–42. https://doi.org/10.1038/s41582-021-00461-4.
Löscher W, Klein P. The feast and famine: epilepsy treatment and treatment gaps in early 21st century. Neuropharmacology. 2020. https://doi.org/10.1016/j.neuropharm.2020.108055.
Yasmen N, Sluter MN, Yu Y, Jiang J. Ganaxolone for management of seizures associated with CDKL5 deficiency disorder. Trends Pharmacol Sci. 2023;44:128–9. https://doi.org/10.1016/j.tips.2022.11.007.
Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–24. https://doi.org/10.1046/j.1528-1157.2001.28900.x.
Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020. https://doi.org/10.1016/j.neuropharm.2020.107966.
Loscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and New innovative treatment options. Pharmacol Rev. 2020;72:606–38. https://doi.org/10.1124/pr.120.019539.
Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75:279–86. https://doi.org/10.1001/jamaneurol.2017.3949.
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–76. https://doi.org/10.1038/jcbfm.2012.84.
Jiang J, Qiu J, Li Q, Shi Z. Prostaglandin E2 signaling: alternative target for glioblastoma? Trends Cancer. 2017;3:75–8. https://doi.org/10.1016/j.trecan.2016.12.002.
Schimmel SJ, Acosta S, Lozano D. Neuroinflammation in traumatic brain injury: a chronic response to an acute injury. Brain Circ. 2017;3:135–42. https://doi.org/10.4103/bc.bc_18_17.
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. https://doi.org/10.1186/s12974-019-1516-2.
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. https://doi.org/10.1186/s40035-020-00221-2.
Alghamri MS, McClellan BL, Hartlage CS, Haase S, Faisal SM, Thalla R, Dabaja A, Banerjee K, Carney SV, Mujeeb AA, Olin MR, Moon JJ, Schwendeman A, Lowenstein PR, Castro MG. Targeting neuroinflammation in brain cancer: uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.680021.
Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signalling cues and cellular infiltration. J Neuroinflammation. 2021;18:284. https://doi.org/10.1186/s12974-021-02337-2.
Jiang J, Yu Y. Small molecules targeting cyclooxygenase/prostanoid cascade in experimental brain ischemia: Do they translate? Med Res Rev. 2021;41:828–57. https://doi.org/10.1002/med.21744.
Khan H, Sharma K, Kumar A, Kaur A, Singh TG. Therapeutic implications of cyclooxygenase (COX) inhibitors in ischemic injury. Inflamm Res. 2022;71:277–92. https://doi.org/10.1007/s00011-022-01546-6.
Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, Brodie MJ, Brooks-Kayal AR, Engel J Jr, Forcelli PA, Hirsch LJ, Kaminski RM, Klitgaard H, Kobow K, Lowenstein DH, Pearl PL, Pitkanen A, Puhakka N, Rogawski MA, Schmidt D, Sillanpaa M, Sloviter RS, Steinhauser C, Vezzani A, Walker MC, Loscher W. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia. 2018;59:37–66. https://doi.org/10.1111/epi.13965.
Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, Henshall DC, Kaufer D, Koh S, Loscher W, Louboutin JP, Mishto M, Norwood BA, Palma E, Poulter MO, Terrone G, Vezzani A, Kaminski RM. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia. 2017;58(Suppl 3):27–38. https://doi.org/10.1111/epi.13783.
Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63:125–49. https://doi.org/10.1016/s0301-0082(00)00022-8.
Dey A, Kang X, Qiu J, Du Y, Jiang J. Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol Sci. 2016;37:463–84. https://doi.org/10.1016/j.tips.2016.03.001.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–53. https://doi.org/10.1111/jnc.13607.
Kan AA, de Jager W, de Wit M, Heijnen C, van Zuiden M, Ferrier C, van Rijen P, Gosselaar P, Hessel E, van Nieuwenhuizen O, de Graan PN. Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. J Neuroinflammation. 2012;9:207. https://doi.org/10.1186/1742-2094-9-207.
Patterson KP, Brennan GP, Curran M, Kinney-Lang E, Dube C, Rashid F, Ly C, Obenaus A, Baram TZ. Rapid, Coordinate Inflammatory Responses after Experimental Febrile Status Epilepticus: Implications for Epileptogenesis. eNeuro 2015;2. https://doi.org/10.1523/ENEURO.0034-15.2015.
Shi LM, Chen RJ, Zhang H, Jiang CM, Gong J. Cerebrospinal fluid neuron specific enolase, interleukin-1beta and erythropoietin concentrations in children after seizures. Childs Nerv Syst. 2017;33:805–11. https://doi.org/10.1007/s00381-017-3359-4.
Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15:144. https://doi.org/10.1186/s12974-018-1192-7.
Levite M. Autoimmune epilepsy. Nat Immunol. 2002;3:500. https://doi.org/10.1038/ni0602-500.
Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, Vincent A, Elger CE. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–44. https://doi.org/10.1212/01.wnl.0000276946.08412.ef.
Michael BD, Solomon T. Seizures and encephalitis: clinical features, management, and potential pathophysiologic mechanisms. Epilepsia. 2012;53(Suppl 4):63–71. https://doi.org/10.1111/j.1528-1167.2012.03615.x.
Geis C, Planaguma J, Carreno M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019;129:926–40. https://doi.org/10.1172/JCI125178.
Sakamoto M, Matsumoto R, Shimotake A, Togawa J, Takeyama H, Kobayashi K, Leypoldt F, Wandinger KP, Kondo T, Takahashi R, Ikeda A. Diagnostic value of an algorithm for autoimmune epilepsy in a retrospective cohort. Front Neurol. 2022. https://doi.org/10.3389/fneur.2022.902157.
Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–47. https://doi.org/10.1093/brain/awl317.
Zattoni M, Mura ML, Deprez F, Schwendener RA, Engelhardt B, Frei K, Fritschy JM. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31:4037–50. https://doi.org/10.1523/JNEUROSCI.6210-10.2011.
Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl 1):26–34. https://doi.org/10.1111/j.1528-1167.2012.03472.x.
Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. https://doi.org/10.1016/j.tins.2013.11.002.
Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. https://doi.org/10.1016/j.bbi.2008.03.009.
Patel DC, Wilcox KS, Metcalf CS. Novel targets for developing antiseizure and potentially. Antiepileptogenic Drugs Epilepsy Curr. 2017;17:293–8. https://doi.org/10.5698/1535-7597.17.5.293.
Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40. https://doi.org/10.1038/nri1664.
Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience. 2006;137:301–8. https://doi.org/10.1016/j.neuroscience.2005.07.063.
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–9. https://doi.org/10.1038/nm.2127.
Bauer S, Cepok S, Todorova-Rudolph A, Nowak M, Koller M, Lorenz R, Oertel WH, Rosenow F, Hemmer B, Hamer HM. Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res. 2009;86:82–8. https://doi.org/10.1016/j.eplepsyres.2009.05.009.
Minami M, Kuraishi Y, Satoh M. Effects of kainic acid on messenger RNA levels of IL-1 beta, IL-6, TNF alpha and LIF in the rat brain. Biochem Biophys Res Commun. 1991;176:593–8. https://doi.org/10.1016/s0006-291x(05)80225-6.
Yabuuchi K, Minami M, Katsumata S, Satoh M. In situ hybridization study of interleukin-1 beta mRNA induced by kainic acid in the rat brain. Brain Res Mol Brain Res. 1993;20:153–61. https://doi.org/10.1016/0169-328x(93)90121-5.
Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–65.
Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):30–5. https://doi.org/10.1046/j.1528-1157.43.s.5.14.x.
Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, Malva J, Randle JC, Allan S, Vezzani A. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47:1160–8. https://doi.org/10.1111/j.1528-1167.2006.00590.x.
Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–15. https://doi.org/10.1007/s13311-011-0039-z.
Zhu G, Okada M, Yoshida S, Mori F, Ueno S, Wakabayashi K, Kaneko S. Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res. 2006;71:107–16. https://doi.org/10.1016/j.eplepsyres.2006.05.017.
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–700. https://doi.org/10.1523/jneurosci.23-25-08692.2003.
Postnikova TY, Zubareva OE, Kovalenko AA, Kim KK, Magazanik LG, Zaitsev AV. Status epilepticus impairs synaptic plasticity in rat hippocampus and is followed by changes in expression of NMDA receptors. Biochemistry (Mosc). 2017;82:282–90. https://doi.org/10.1134/S0006297917030063.
Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292:497–504.
Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. https://doi.org/10.1016/s1074-7613(01)00151-0.
Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. https://doi.org/10.1186/ar1917.
Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–24. https://doi.org/10.1517/14728222.11.5.613.
Gruol DL. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015;96:42–54. https://doi.org/10.1016/j.neuropharm.2014.10.023.
Billiau AD, Witters P, Ceulemans B, Kasran A, Wouters C, Lagae L. Intravenous immunoglobulins in refractory childhood-onset epilepsy: effects on seizure frequency, EEG activity, and cerebrospinal fluid cytokine profile. Epilepsia. 2007;48:1739–49. https://doi.org/10.1111/j.1528-1167.2007.01134.x.
Hulkkonen J, Koskikallio E, Rainesalo S, Keranen T, Hurme M, Peltola J. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Res. 2004;59:199–205. https://doi.org/10.1016/j.eplepsyres.2004.04.007.
Peltola J, Hurme M, Miettinen A, Keranen T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Res. 1998;31:129–33. https://doi.org/10.1016/s0920-1211(98)00024-2.
Nowak M, Bauer S, Haag A, Cepok S, Todorova-Rudolph A, Tackenberg B, Norwood B, Oertel WH, Rosenow F, Hemmer B, Hamer HM. Interictal alterations of cytokines and leukocytes in patients with active epilepsy. Brain Behav Immun. 2011;25:423–8. https://doi.org/10.1016/j.bbi.2010.10.022.
Peltola J, Palmio J, Korhonen L, Suhonen J, Miettinen A, Hurme M, Lindholm D, Keranen T. Interleukin-6 and interleukin-1 receptor antagonist in cerebrospinal fluid from patients with recent tonic-clonic seizures. Epilepsy Res. 2000;41:205–11. https://doi.org/10.1016/s0920-1211(00)00140-6.
Alapirtti T, Rinta S, Hulkkonen J, Makinen R, Keranen T, Peltola J. Interleukin-6, interleukin-1 receptor antagonist and interleukin-1beta production in patients with focal epilepsy: a video-EEG study. J Neurol Sci. 2009;280:94–7. https://doi.org/10.1016/j.jns.2009.02.355.
Alapirtti T, Lehtimaki K, Nieminen R, Makinen R, Raitanen J, Moilanen E, Makinen J, Peltola J. The production of IL-6 in acute epileptic seizure: A video-EEG study. J Neuroimmunol. 2018;316:50–5. https://doi.org/10.1016/j.jneuroim.2017.12.008.
de Bock F, Dornand J, Rondouin G. Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. NeuroReport. 1996;7:1125–9. https://doi.org/10.1097/00001756-199604260-00004.
Lehtimaki KA, Peltola J, Koskikallio E, Keranen T, Honkaniemi J. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110:253–60. https://doi.org/10.1016/s0169-328x(02)00654-x.
De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–33. https://doi.org/10.1046/j.1460-9568.2000.00140.x.
Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M. Induction of interleukin-6 by depolarization of neurons. J Neurosci. 2000;20:8637–42. https://doi.org/10.1523/jneurosci.20-23-08637.2000.
Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. https://doi.org/10.1111/epi.12960.
Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–5. https://doi.org/10.1073/pnas.90.21.10061.
Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–87. https://doi.org/10.1002/jnr.10635.
Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365:106–10. https://doi.org/10.1016/j.neulet.2004.04.061.
De Sarro G, Russo E, Ferreri G, Giuseppe B, Flocco MA, Di Paola ED, De Sarro A. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol Biochem Behav. 2004;77:761–6. https://doi.org/10.1016/j.pbb.2004.01.012.
Penkowa M, Molinero A, Carrasco J, Hidalgo J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience. 2001;102:805–18. https://doi.org/10.1016/s0306-4522(00)00515-7.
Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–20. https://doi.org/10.1016/j.semnephrol.2007.02.009.
Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32:236–43. https://doi.org/10.1016/j.semnephrol.2012.04.002.
Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16:1371–83. https://doi.org/10.1681/ASN.2004121070.
Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. https://doi.org/10.1111/j.1460-9568.2005.04539.x.
Lu Y, Xue T, Yuan J, Li Y, Wu Y, Xi Z, Xiao Z, Chen Y, Wang X. Increased expression of TGFbeta type I receptor in brain tissues of patients with temporal lobe epilepsy. Clin Sci (Lond). 2009;117:17–22. https://doi.org/10.1042/CS20080347.
Yu W, Zou Y, Du Y, Luo J, Zhang M, Yang W, Wang X, Lu Y. Altered cerebrospinal fluid concentrations of TGFbeta1 in patients with drug-resistant epilepsy. Neurochem Res. 2014;39:2211–7. https://doi.org/10.1007/s11064-014-1422-z.
Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000;75:248–58. https://doi.org/10.1016/s0169-328x(99)00306-x.
Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–44. https://doi.org/10.1046/j.1460-9568.2000.00131.x.
van Vliet EA, da Costa AS, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–34. https://doi.org/10.1093/brain/awl318.
Salar S, Maslarova A, Lippmann K, Nichtweiss J, Weissberg I, Sheintuch L, Kunz WS, Shorer Z, Friedman A, Heinemann U. Blood-brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 2014;55:1255–63. https://doi.org/10.1111/epi.12713.
Bar-Klein G, Cacheaux LP, Kamintsky L, Prager O, Weissberg I, Schoknecht K, Cheng P, Kim SY, Wood L, Heinemann U, Kaufer D, Friedman A. Losartan prevents acquired epilepsy via TGF-beta signaling suppression. Ann Neurol. 2014;75:864–75. https://doi.org/10.1002/ana.24147.
Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–35. https://doi.org/10.1523/JNEUROSCI.0430-09.2009.
Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone C, Becker A, Frigerio F, Vezzani A, Buckwalter MS, Huguenard JR, Friedman A, Kaufer D. Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis. 2015;78:115–25. https://doi.org/10.1016/j.nbd.2015.02.029.
Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014. https://doi.org/10.1155/2014/861231.
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–8. https://doi.org/10.1074/jbc.M600504200.
Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–25. https://doi.org/10.1016/j.yfrne.2011.12.002.
Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–28. https://doi.org/10.1523/JNEUROSCI.4486-04.2005.
Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1995;92:11294–8. https://doi.org/10.1073/pnas.92.24.11294.
Savin C, Triesch J, Meyer-Hermann M. Epileptogenesis due to glia-mediated synaptic scaling. J R Soc Interface. 2009;6:655–68. https://doi.org/10.1098/rsif.2008.0387.
Shandra AA, Godlevsky LS, Vastyanov RS, Oleinik AA, Konovalenko VL, Rapoport EN, Korobka NN. The role of TNF-alpha in amygdala kindled rats. Neurosci Res. 2002;42:147–53. https://doi.org/10.1016/s0168-0102(01)00309-1.
Godlevsky LS, Shandra AA, Oleinik AA, Vastyanov RS, Kostyushov VV, Timchishin OL. TNF-alpha in cerebral cortex and cerebellum is affected by amygdalar kindling but not by stimulation of cerebellum. Pol J Pharmacol. 2002;54:655–60.
Yuhas Y, Weizman A, Ashkenazi S. Bidirectional concentration-dependent effects of tumor necrosis factor alpha in Shigella dysenteriae-related seizures. Infect Immun. 2003;71:2288–91. https://doi.org/10.1128/IAI.71.4.2288-2291.2003.
Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–12. https://doi.org/10.1002/ana.20480.
Shinoda S, Skradski SL, Araki T, Schindler CK, Meller R, Lan JQ, Taki W, Simon RP, Henshall DC. Formation of a tumour necrosis factor receptor 1 molecular scaffolding complex and activation of apoptosis signal-regulating kinase 1 during seizure-induced neuronal death. Eur J Neurosci. 2003;17:2065–76. https://doi.org/10.1046/j.1460-9568.2003.02655.x.
Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–5. https://doi.org/10.1073/pnas.95.2.570.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. https://doi.org/10.1038/nature00858.
Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–43. https://doi.org/10.1111/j.1365-2796.2003.01296.x.
Walker L, Tse K, Ricci E, Thippeswamy T, Sills GJ, White SH, Antoine DJ, Marson A, Pirmohamed M. High mobility group box 1 in the inflammatory pathogenesis of epilepsy: profiling circulating levels after experimental and clinical seizures. The Lancet. 2014;383:S105.
Kan M, Song L, Zhang X, Zhang J, Fang P. Circulating high mobility group box-1 and toll-like receptor 4 expressions increase the risk and severity of epilepsy. Braz J Med Biol Res. 2019. https://doi.org/10.1590/1414-431X20197374.
Han Y, Yang L, Liu X, Feng Y, Pang Z, Lin Y. HMGB1/CXCL12-Mediated immunity and Th17 cells might underlie highly suspected autoimmune epilepsy in elderly individuals. Neuropsychiatr Dis Treat. 2020;16:1285–93. https://doi.org/10.2147/NDT.S242766.
Zhao J, Zheng Y, Liu K, Chen J, Lai N, Fei F, Shi J, Xu C, Wang S, Nishibori M, Wang Y, Chen Z. HMGB1 Is a therapeutic target and biomarker in diazepam-refractory status epilepticus with wide time window. Neurotherapeutics. 2020;17:710–21. https://doi.org/10.1007/s13311-019-00815-3.
Zhao J, Wang Y, Xu C, Liu K, Wang Y, Chen L, Wu X, Gao F, Guo Y, Zhu J, Wang S, Nishibori M, Chen Z. Therapeutic potential of an anti-high mobility group box-1 monoclonal antibody in epilepsy. Brain Behav Immun. 2017;64:308–19. https://doi.org/10.1016/j.bbi.2017.02.002.
Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G. The inflammatory molecules IL-1beta and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci. 2014;8:155. https://doi.org/10.3389/fncel.2014.00155.
Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal. 2014;21:1726–40. https://doi.org/10.1089/ars.2013.5349.
Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285:2944–71. https://doi.org/10.1111/febs.14466.
Wu Y, Wang X, Mo X, Xi Z, Xiao F, Li J, Zhu X, Luan G, Wang Y, Li Y, Zhang J. Expression of monocyte chemoattractant protein-1 in brain tissue of patients with intractable epilepsy. Clin Neuropathol. 2008;27:55–63. https://doi.org/10.5414/npp27055.
Choi J, Nordli DR Jr, Alden TD, DiPatri A Jr, Laux L, Kelley K, Rosenow J, Schuele SU, Rajaram V, Koh S. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J Neuroinflammation. 2009;6:38. https://doi.org/10.1186/1742-2094-6-38.
van Gassen KL, de Wit M, Koerkamp MJ, Rensen MG, van Rijen PC, Holstege FC, Lindhout D, de Graan PN. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49:1055–65. https://doi.org/10.1111/j.1528-1167.2007.01470.x.
Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, Zhou LJ, Mogilevsky R, Wang W, Wu LJ. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1beta production after status Epilepticus. J Neurosci. 2017;37:7878–92. https://doi.org/10.1523/JNEUROSCI.0315-17.2017.
Foresti ML, Arisi GM, Katki K, Montanez A, Sanchez RM, Shapiro LA. Chemokine CCL2 and its receptor CCR2 are increased in the hippocampus following pilocarpine-induced status epilepticus. J Neuroinflammation. 2009;6:40. https://doi.org/10.1186/1742-2094-6-40.
Xu JH, Long L, Tang YC, Zhang JT, Hut HT, Tang FR. CCR3, CCR2A and macrophage inflammatory protein (MIP)-1a, monocyte chemotactic protein-1 (MCP-1) in the mouse hippocampus during and after pilocarpine-induced status epilepticus (PISE). Neuropathol Appl Neurobiol. 2009;35:496–514. https://doi.org/10.1111/j.1365-2990.2009.01022.x.
Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, Dingledine R. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A. 2016;113:E5665–74. https://doi.org/10.1073/pnas.1604263113.
Cerri C, Genovesi S, Allegra M, Pistillo F, Puntener U, Guglielmotti A, Perry VH, Bozzi Y, Caleo M. The Chemokine CCL2 mediates the seizure-enhancing effects of systemic inflammation. J Neurosci. 2016;36:3777–88. https://doi.org/10.1523/JNEUROSCI.0451-15.2016.
Mennicken F, Chabot JG, Quirion R. Systemic administration of kainic acid in adult rat stimulates expression of the chemokine receptor CCR5 in the forebrain. Glia. 2002;37:124–38. https://doi.org/10.1002/glia.10021.
Chen Z, Yu S, Bakhiet M, Winblad B, Zhu J. The chemokine receptor CCR5 is not a necessary inflammatory mediator in kainic acid-induced hippocampal injury: evidence for a compensatory effect by increased CCR2 and CCR3. J Neurochem. 2003;86:61–8. https://doi.org/10.1046/j.1471-4159.2003.01807.x.
Louboutin JP, Chekmasova A, Marusich E, Agrawal L, Strayer DS. Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB J. 2011;25:737–53. https://doi.org/10.1096/fj.10-161851.
Xu Y, Zeng K, Han Y, Wang L, Chen D, Xi Z, Wang H, Wang X, Chen G. Altered expression of CX3CL1 in patients with epilepsy and in a rat model. Am J Pathol. 2012;180:1950–62. https://doi.org/10.1016/j.ajpath.2012.01.024.
Yeo SI, Kim JE, Ryu HJ, Seo CH, Lee BC, Choi IG, Kim DS, Kang TC. The roles of fractalkine/CX3CR1 system in neuronal death following pilocarpine-induced status epilepticus. J Neuroimmunol. 2011;234:93–102. https://doi.org/10.1016/j.jneuroim.2011.03.005.
Roseti C, Fucile S, Lauro C, Martinello K, Bertollini C, Esposito V, Mascia A, Catalano M, Aronica E, Limatola C, Palma E. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia. 2013;54:1834–44. https://doi.org/10.1111/epi.12354.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. https://doi.org/10.1146/annurev.pharmtox.38.1.97.
Mancini A, Jovanovic DV, He QW, Di Battista JA. Site-specific proteolysis of cyclooxygenase-2: a putative step in inflammatory prostaglandin E(2) biosynthesis. J Cell Biochem. 2007;101:425–41. https://doi.org/10.1002/jcb.21191.
Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Cyclooxygenase pathways. Acta Biochim Pol. 2014;61:639–49.
Faour WH, Alaaeddine N, Mancini A, He QW, Jovanovic D, Di Battista JA. Early growth response factor-1 mediates prostaglandin E2-dependent transcriptional suppression of cytokine-induced tumor necrosis factor-alpha gene expression in human macrophages and rheumatoid arthritis-affected synovial fibroblasts. J Biol Chem. 2005;280:9536–46. https://doi.org/10.1074/jbc.M414067200.
Mancini AD, Di Battista JA. The cardinal role of the phospholipase A(2)/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E(2) (PCPP) axis in inflammostasis. Inflamm Res. 2011;60:1083–92. https://doi.org/10.1007/s00011-011-0385-7.
Hartings JA, York J, Carroll CP, Hinzman JM, Mahoney E, Krueger B, Winkler MKL, Major S, Horst V, Jahnke P, Woitzik J, Kola V, Du Y, Hagen M, Jiang J, Dreier JP. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. 2017;140:2673–90. https://doi.org/10.1093/brain/awx214.
Kang X, Qiu J, Li Q, Bell KA, Du Y, Jung DW, Lee JY, Hao J, Jiang J. Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin E2 receptor EP2 subtype. Sci Rep. 2017;7:9459. https://doi.org/10.1038/s41598-017-09528-z.
Qiu J, Shi Z, Jiang J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov Today. 2017;22:148–56. https://doi.org/10.1016/j.drudis.2016.09.017.
Qiu J, Li Q, Bell KA, Yao X, Du Y, Zhang E, Yu JJ, Yu Y, Shi Z, Jiang J. Small-molecule inhibition of prostaglandin E receptor 2 impairs cyclooxygenase-associated malignant glioma growth. Br J Pharmacol. 2019;176:1680–99. https://doi.org/10.1111/bph.14622.
Yu Y, Nguyen DT, Jiang J. G protein-coupled receptors in acquired epilepsy: Druggability and translatability. Prog Neurobiol. 2019. https://doi.org/10.1016/j.pneurobio.2019.101682.
Hou R, Yu Y, Sluter MN, Li L, Hao J, Fang J, Yang J, Jiang J. Targeting EP2 receptor with multifaceted mechanisms for high-risk neuroblastoma. Cell Rep. 2022. https://doi.org/10.1016/j.celrep.2022.111000.
Hou R, Yu Y, Jiang J. Prostaglandin E2 in neuroblastoma: Targeting synthesis or signaling? Biomedicine Pharmacotherapy. 2022. https://doi.org/10.1016/j.biopha.2022.113966.
Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, Rose C, Butterworth RF. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem Int. 2003;42:299–303. https://doi.org/10.1016/s0197-0186(02)00101-8.
Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–16. https://doi.org/10.1016/s1098-8823(03)00040-6.
Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp Neurol. 2003;179:167–75. https://doi.org/10.1016/s0014-4886(02)00019-5.
Du Y, Kemper T, Qiu J, Jiang J. Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert Rev Neurother. 2016;16:123–30. https://doi.org/10.1586/14737175.2016.1134322.
Dhir A. An update of cyclooxygenase (COX)-inhibitors in epilepsy disorders. Expert Opin Investig Drugs. 2019;28:191–205. https://doi.org/10.1080/13543784.2019.1557147.
Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, Castelli M, Machado P, Bohrer D, Veiga M, Ferreira J, Cavalheiro EA, Mello CF. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79:14–21. https://doi.org/10.1016/j.eplepsyres.2007.12.008.
Akula KK, Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: possible involvement of adenosinergic mechanism. Epilepsy Res. 2008;78:60–70. https://doi.org/10.1016/j.eplepsyres.2007.10.008.
Claycomb RJ, Hewett SJ, Hewett JA. Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition. Epilepsia. 2011;52:273–83. https://doi.org/10.1111/j.1528-1167.2010.02889.x.
Kunz T, Oliw EH. Nimesulide aggravates kainic acid-induced seizures in the rat. Pharmacol Toxicol. 2001;88:271–6. https://doi.org/10.1034/j.1600-0773.2001.d01-116.x.
Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13:569–75. https://doi.org/10.1046/j.1460-9568.2001.01420.x.
Polascheck N, Bankstahl M, Loscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2010;224:219–33. https://doi.org/10.1016/j.expneurol.2010.03.014.
Holtman L, van Vliet EA, van Schaik R, Queiroz CM, Aronica E, Gorter JA. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84:56–66. https://doi.org/10.1016/j.eplepsyres.2008.12.006.
Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010;91:49–56. https://doi.org/10.1016/j.eplepsyres.2010.06.011.
Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010;91:104–12. https://doi.org/10.1016/j.prostaglandins.2009.04.003.
Grosser T, Yu Y, Fitzgerald GA. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. https://doi.org/10.1146/annurev-med-011209-153129.
Ikeda-Matsuo Y. The Role of mPGES-1 in Inflammatory Brain Diseases. Biol Pharm Bull. 2017;40:557–63. https://doi.org/10.1248/bpb.b16-01026.
Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:11790–5. https://doi.org/10.1073/pnas.0604400103.
O’Banion MK. Prostaglandin E2 synthases in neurologic homeostasis and disease. Prostaglandins Other Lipid Mediat. 2010;91:113–7. https://doi.org/10.1016/j.prostaglandins.2009.04.008.
Li L, Yasmen N, Hou R, Yang S, Lee JY, Hao J, Yu Y, Jiang J. inducible prostaglandin E synthase as a pharmacological target for ischemic stroke. Neurotherapeutics. 2022;19:366–85. https://doi.org/10.1007/s13311-022-01191-1.
Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–6. https://doi.org/10.4049/jimmunol.168.11.5811.
Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Akira S. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–48.
Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–10. https://doi.org/10.1016/j.neures.2006.06.003.
Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis. 2015;76:126–36. https://doi.org/10.1016/j.nbd.2014.12.032.
Yu Y, Jiang J. COX-2/PGE2 axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open. 2020;5:418–31. https://doi.org/10.1002/epi4.12409.
Takemiya T, Matsumura K, Sugiura H, Maehara M, Yasuda S, Uematsu S, Akira S, Yamagata K. Endothelial microsomal prostaglandin E synthase-1 exacerbates neuronal loss induced by kainate. J Neurosci Res. 2010;88:381–90. https://doi.org/10.1002/jnr.22195.
Yasmen N, Sluter MN, Li L, Yu Y, Jiang J. Transient inhibition of microsomal prostaglandin E synthase-1 after status epilepticus blunts brain inflammation and is neuroprotective. Mol Brain. 2023. https://doi.org/10.1186/s13041-023-01008-y.
Shimada T, Takemiya T, Sugiura H, Yamagata K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediators Inflamm. 2014. https://doi.org/10.1155/2014/901902.
Takemiya T, Matsumura K, Sugiura H, Yasuda S, Uematsu S, Akira S, Yamagata K. Endothelial microsomal prostaglandin E synthase-1 facilitates neurotoxicity by elevating astrocytic Ca2+ levels. Neurochem Int. 2011;58:489–96. https://doi.org/10.1016/j.neuint.2011.01.003.
Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–53. https://doi.org/10.1124/mol.107.041210.
Feldmann M, Asselin MC, Liu J, Wang S, McMahon A, Anton-Rodriguez J, Walker M, Symms M, Brown G, Hinz R, Matthews J, Bauer M, Langer O, Thom M, Jones T, Vollmar C, Duncan JS, Sisodiya SM, Koepp MJ. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013;12:777–85. https://doi.org/10.1016/S1474-4422(13)70109-1.
Zibell G, Unkruer B, Pekcec A, Hartz AM, Bauer B, Miller DS, Potschka H. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology. 2009;56:849–55. https://doi.org/10.1016/j.neuropharm.2009.01.009.
Schlichtiger J, Pekcec A, Bartmann H, Winter P, Fuest C, Soerensen J, Potschka H. Celecoxib treatment restores pharmacosensitivity in a rat model of pharmacoresistant epilepsy. Br J Pharmacol. 2010;160:1062–71. https://doi.org/10.1111/j.1476-5381.2010.00765.x.
Soldner ELB, Hartz AMS, Akanuma SI, Pekcec A, Doods H, Kryscio RJ, Hosoya KI, Bauer B. Inhibition of human microsomal PGE2 synthase-1 reduces seizure-induced increases of P-glycoprotein expression and activity at the blood-brain barrier. FASEB J. 2019;33:13966–81. https://doi.org/10.1096/fj.201901460RR.
Ahmad AS, Saleem S, Ahmad M, Dore S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89:265–70. https://doi.org/10.1093/toxsci/kfj022.
Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–9. https://doi.org/10.1038/nm1362.
Li L, Sluter MN, Yu Y, Jiang J. Prostaglandin E receptors as targets for ischemic stroke: Novel evidence and molecular mechanisms of efficacy. Pharmacol Res. 2021. https://doi.org/10.1016/j.phrs.2020.105238.
Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Royes LF, Ferreira J, Calixto JB, Mello CF. Modulation of pentylenetetrazol-induced seizures by prostaglandin E2 receptors. Neuroscience. 2008;152:1110–8. https://doi.org/10.1016/j.neuroscience.2008.01.005.
Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Royes LF, Ferreira J, Calixto JB, Otalora LF, Garrido-Sanabria ER, Mello CF. Prostaglandin E2 modulates Na+, K+-ATPase activity in rat hippocampus: implications for neurological diseases. J Neurochem. 2009;109:416–26. https://doi.org/10.1111/j.1471-4159.2009.05961.x.
Reschke CR, Poersch AB, Masson CJ, Jesse AC, Marafiga JR, Lenz QF, Oliveira MS, Henshall DC, Mello CF. Systemic delivery of selective EP1 and EP3 receptor antagonists attenuates pentylenetetrazole-induced seizures in mice. Int J Physiol Pathophysiol Pharmacol. 2018;10:47–59.
Collins SA, Huff C, Chiaia N, Gudelsky GA, Yamamoto BK. 3,4-methylenedioxymethamphetamine increases excitability in the dentate gyrus: role of 5HT2A receptor-induced PGE2 signaling. J Neurochem. 2016;136:1074–84. https://doi.org/10.1111/jnc.13493.
Fischborn SV, Soerensen J, Potschka H. Targeting the prostaglandin E2 EP1 receptor and cyclooxygenase-2 in the amygdala kindling model in mice. Epilepsy Res. 2010;91:57–65. https://doi.org/10.1016/j.eplepsyres.2010.06.012.
Rojas A, Gueorguieva P, Lelutiu N, Quan Y, Shaw R, Dingledine R. The prostaglandin EP1 receptor potentiates kainate receptor activation via a protein kinase C pathway and exacerbates status epilepticus. Neurobiol Dis. 2014;70:74–89. https://doi.org/10.1016/j.nbd.2014.06.004.
Pekcec A, Unkruer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther. 2009;330:939–47. https://doi.org/10.1124/jpet.109.152520.
Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34:413–23. https://doi.org/10.1016/j.tips.2013.05.003.
Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumont F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100:6736–40. https://doi.org/10.1073/pnas.1037343100.
McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–68. https://doi.org/10.1523/JNEUROSCI.4485-03.2004.
Elberg G, Elberg D, Lewis TV, Guruswamy S, Chen L, Logan CJ, Chan MD, Turman MA. EP2 receptor mediates PGE2-induced cystogenesis of human renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1622–32. https://doi.org/10.1152/ajprenal.00036.2007.
Jiang J, Ganesh T, Du Y, Thepchatri P, Rojas A, Lewis I, Kurtkaya S, Li L, Qui M, Serrano G, Shaw R, Sun A, Dingledine R. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A. 2010;107:2307–12. https://doi.org/10.1073/pnas.0909310107.
Jiang J, Van TM, Ganesh T, Dingledine R. Discovery of 2-piperidinyl phenyl benzamides and trisubstituted pyrimidines as positive allosteric modulators of the prostaglandin receptor EP2. ACS Chem Neurosci. 2018;9:699–707. https://doi.org/10.1021/acschemneuro.7b00486.
Liu Q, Liang X, Wang Q, Wilson EN, Lam R, Wang J, Kong W, Tsai C, Pan T, Larkin PB, Shamloo M, Andreasson KI. PGE2 signaling via the neuronal EP2 receptor increases injury in a model of cerebral ischemia. Proc Natl Acad Sci U S A. 2019;116:10019–24. https://doi.org/10.1073/pnas.1818544116.
Hou R, Yu Y, Jiang J. PGE2 receptors in detrusor muscle: drugging the undruggable for urgency. Biochem Pharmacol. 2021. https://doi.org/10.1016/j.bcp.2020.114363.
Quan Y, Jiang J, Dingledine R. EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem. 2013;288:9293–302. https://doi.org/10.1074/jbc.M113.455816.
Fu Y, Yang MS, Jiang J, Ganesh T, Joe E, Dingledine R. EP2 receptor signaling regulates microglia death. Mol Pharmacol. 2015;88:161–70. https://doi.org/10.1124/mol.115.098202.
Sluter MN, Hou R, Li L, Yasmen N, Yu Y, Liu J, Jiang J. EP2 antagonists (2011–2021): a decade’s journey from discovery to therapeutics. J Med Chem. 2021;64:11816–36. https://doi.org/10.1021/acs.jmedchem.1c00816.
Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, Speigel I, Rojas A, Lelutiu N, Dingledine R. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012;109:3149–54. https://doi.org/10.1073/pnas.1120195109.
Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110:3591–6. https://doi.org/10.1073/pnas.1218498110.
Rojas A, Ganesh T, Lelutiu N, Gueorguieva P, Dingledine R. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology. 2015;93:15–27. https://doi.org/10.1016/j.neuropharm.2015.01.017.
Rojas A, Ganesh T, Manji Z, O’Neill T, Dingledine R. Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats. Neuropharmacology. 2016;110:419–30. https://doi.org/10.1016/j.neuropharm.2016.07.028.
Rojas A, Ganesh T, Wang W, Wang J, Dingledine R. A rat model of organophosphate-induced status epilepticus and the beneficial effects of EP2 receptor inhibition. Neurobiol Dis. 2020. https://doi.org/10.1016/j.nbd.2019.02.010.
Jiang J, Yu Y, Kinjo ER, Du Y, Nguyen HP, Dingledine R. Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology. 2019;149:149–60. https://doi.org/10.1016/j.neuropharm.2019.02.011.
Varvel NH, Espinosa-Garcia C, Hunter-Chang S, Chen D, Biegel A, Hsieh A, Blackmer-Raynolds L, Ganesh T, Dingledine R. peripheral myeloid cell EP2 activation contributes to the deleterious consequences of status epilepticus. J Neurosci. 2021;41:1105–17. https://doi.org/10.1523/JNEUROSCI.2040-20.2020.
Marchi N. Experimental status epilepticus, COX-2 and BDNF: Connecting the dots. Epilepsia Open. 2021;6:466–7. https://doi.org/10.1002/epi4.12501.
Gu B, Huang YZ, He XP, Joshi RB, Jang W, McNamara JO. A Peptide uncoupling BDNF receptor TrkB from phospholipase Cgamma1 prevents epilepsy induced by status epilepticus. Neuron. 2015;88:484–91. https://doi.org/10.1016/j.neuron.2015.09.032.
Lin TW, Harward SC, Huang YZ, McNamara JO. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology. 2020. https://doi.org/10.1016/j.neuropharm.2019.107734.
Amaradhi R, Mohammed S, Banik A, Franklin R, Dingledine R, Ganesh T. Second-generation prostaglandin receptor EP2 antagonist, TG8-260, with high potency, selectivity, oral bioavailability, and anti-inflammatory properties. ACS Pharmacol Transl Sci. 2022;5:118–33. https://doi.org/10.1021/acsptsci.1c00255.
Rojas A, Amaradhi R, Banik A, Jiang C, Abreu-Melon J, Wang S, Dingledine R, Ganesh T. A novel second-generation EP2 receptor antagonist reduces neuroinflammation and gliosis after status epilepticus in rats. Neurotherapeutics. 2021;18:1207–25. https://doi.org/10.1007/s13311-020-00969-5.
Rawat V, Eastman CL, Amaradhi R, Banik A, Fender JS, Dingledine RJ, D’Ambrosio R, Ganesh T. Temporal expression of neuroinflammatory and oxidative stress markers and prostaglandin E2 receptor EP2 antagonist effect in a rat model of epileptogenesis. ACS Pharmacol Transl Sci. 2023;6:128–38. https://doi.org/10.1021/acsptsci.2c00189.
Varvel NH, Amaradhi R, Espinosa-Garcia C, Duddy S, Franklin R, Banik A, Aleman-Ruiz C, Blackmar-Raynolds L, Wang W, Honore T, Ganesh T, Dingledine R. Preclinical development of an EP2 antagonist for post-seizure cognitive deficits. Neuropharmacology. 2023. https://doi.org/10.1016/j.neuropharm.2022.109356.
Nagib MM, Yu Y, Jiang J. Targeting prostaglandin receptor EP2 for adjunctive treatment of status epilepticus. Pharmacol Ther. 2020. https://doi.org/10.1016/j.pharmthera.2020.107504.
Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc Natl Acad Sci U S A. 1999;96:10501–6. https://doi.org/10.1073/pnas.96.18.10501.
Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–20. https://doi.org/10.1038/5583.
Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–45. https://doi.org/10.1172/JCI6579.
Savonenko A, Munoz P, Melnikova T, Wang Q, Liang X, Breyer RM, Montine TJ, Kirkwood A, Andreasson K. Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Exp Neurol. 2009;217:63–73. https://doi.org/10.1016/j.expneurol.2009.01.016.
Yang H, Zhang J, Breyer RM, Chen C. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem. 2009;108:295–304. https://doi.org/10.1111/j.1471-4159.2008.05766.x.
Rawat V, Banik A, Amaradhi R, Rojas A, Taval S, Nagy T, Dingledine R, Ganesh T. Pharmacological antagonism of EP2 receptor does not modify basal cardiovascular and respiratory function, blood cell counts, and bone morphology in animal models. Biomed Pharmacother. 2022. https://doi.org/10.1016/j.biopha.2022.112646.
Fabisiak T, Patel M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.976953.
Almeida C, Pongilio RP, Movio MI, Higa GSV, Resende RR, Jiang J, Kinjo ER, Kihara AH. Distinct cell-specific roles of NOX2 and MyD88 in epileptogenesis. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.926776.
Galanopoulou AS, Loscher W, Lubbers L, O’Brien TJ, Staley K, Vezzani A, D’Ambrosio R, White HS, Sontheimer H, Wolf JA, Twyman R, Whittemore V, Wilcox KS, Klein B. Antiepileptogenesis and disease modification: progress, challenges, and the path forward-report of the preclinical working group of the 2018 NINDS-sponsored antiepileptogenesis and disease modification workshop. Epilepsia Open. 2021;6:276–96. https://doi.org/10.1002/epi4.12490.
Walker LE, Sills GJ, Jorgensen A, Alapirtti T, Peltola J, Brodie MJ, Marson AG, Vezzani A, Pirmohamed M. High-mobility group box 1 as a predictive biomarker for drug-resistant epilepsy: a proof-of-concept study. Epilepsia. 2022;63:e1–6. https://doi.org/10.1111/epi.17116.
Ravizza T, Terrone G, Salamone A, Frigerio F, Balosso S, Antoine DJ, Vezzani A. High mobility group box 1 is a novel pathogenic factor and a mechanistic biomarker for epilepsy. Brain Behav Immun. 2018;72:14–21. https://doi.org/10.1016/j.bbi.2017.10.008.
Acknowledgements
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS100947 (J.J.), R21NS109687 (J.J.), and R61NS124923 (J.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors apologize for not being able to cite and discuss all publications that are relevant to the broad topics in this review because of the space limitation.
Author information
Authors and Affiliations
Contributions
Conceptualization: YC, MMN, YY, and JJ; literature search, data collection, and writing: YC and MMN; review and editing: NY, MNS, TLL, YY, and JJ. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Nagib, M.M., Yasmen, N. et al. Neuroinflammatory mediators in acquired epilepsy: an update. Inflamm. Res. 72, 683–701 (2023). https://doi.org/10.1007/s00011-023-01700-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01700-8