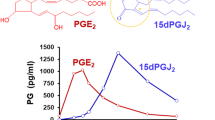
Abstract
The process of inflammation is regulated in part by bioactive lipids of which prostaglandins/eicosanoids form an important class. We provide evidence that the phospholipase A2/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E2 (PCPP) axis is positioned at the core of a natural regulatory circuit controlling the initiation, magnitude, duration, and resolution of the inflammatory response. During the inflammatory phase, proinflammatory cytokine, chemokine and matrix destructive metalloprotease expression levels are moderated by the PCPP axis through the modulation of signaling pathways that control proinflammatory gene expression at transcriptional, post-transcriptional, and translational levels. The PCPP axis also contributes to the activation of lipid mediator class switching; this highly coordinated process results in the biosynthesis of lipoxins and resolvins that promote inflammatory resolution through a variety of cellular and molecular mechanisms. The PCPP axis activity is autoregulated by way of a positive feedback circuit involving PGE2-mediated, p38 MAPK-dependent stabilization of COX-2 mRNA and COX-2 catalytic potentiation via its limited proteolytic cleavage (e.g., Ca2+-activated calpains). In conclusion, through its fine temporal modulation of multiple signaling cassettes via EP1-EP4 GPCRs, PGE2 influences the onset, course, magnitude, and duration of the inflammatory response and functions as a key feedback regulator of the cellular and molecular processes controlling inflammation.

Similar content being viewed by others
Notes
Other enzymes also participate in the production of PGE2. Under basal (i.e., non-inflammatory) conditions, the constitutively and ubiquitously expressed COX-1 isoform catalyzes the rate-limiting step in PGE2 biosynthesis. Basal PGE2 production is required for homeostatic purposes (in the kidney and gastrointestinal tract, for example). The ubiquitous and constitutively expressed cytosolic prostaglandin E synthase (cPGES) preferentially couples to COX-1 for basal PGE2 biosynthesis. Interestingly, a third PGE synthase, namely mPGES-2, has been shown to couple to both COX isoforms with a modest preference for COX-2 [14]. It has been inferred that mPGES-2 may contribute to PGE2 production during the resolution phase of inflammation [15] and/or to homeostatic PGE2 biosynthesis.
Fibroblast-like cells of the synovial membrane. These cells are central orchestrators of rheumatoid arthritis (RA) pathophysiology and a principal source of COX-2/PGE2 axis activity in the RA joint. Synovial fibroblast activation is a defining step in the development of RA as it leads to the formation of the hyperplastic, bone- and cartilage-eroding pannus.
References
Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55.
Ryan GB, Majno G. Acute inflammation. Am J Pathol. 1977;86:185–274.
Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest. 1988;59:200–13.
Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:24–9.
Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–76.
Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–46.
Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999;190:1909–14.
Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21.
Krakauer T. Molecular therapeutic targets in inflammation: cyclooxygenase and NF -kappaB. Curr Drug Targets Inflamm Allergy. 2004;3:317–24.
Di Battista JA, He QW, Zhai B, Mancini A. Modulation of the inflammatory and catabolic response by prostaglandin E2 (PGE2) is partially dependent on the induction of dual specificity phosphatase 1 (DUSP-1) in vitro and in vivo. Inflamm Res. 2009;58(Suppl 2):S97.
Faour WH, Alaaeddine N, Mancini A, He QW, Jovanovic D, Di Battista JA. Early growth response factor-1 mediates prostaglandin E2-dependent transcriptional suppression of cytokine-induced tumor necrosis factor-α gene expression in human macrophages and rheumatoid arthritis-affected synovial fibroblasts. J Biol Chem. 2005;280:9536–46.
Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002;303:1001–6.
He W, Pelletier JP, Martel-Pelletier J, Laufer S, Di Battista JA. Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J Rheumatol. 2002;29:546–53.
Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, Ishii T, Ohmiya Y, Watanabe K, Kudo I. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278:37937–47.
Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–26.
Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol. 1984;2:335–57.
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat New Biol. 1971;231:232–5.
Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–18.
Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9.
Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–41.
Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701.
Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol. 2009;461:379–96.
Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104:20979–84.
Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Antiinflammatory cyclopentenone prostaglandins are direct inhibitors of Ikappa B kinase. Nature. 2000;403:103–8.
Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12, 14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–9.
Bell-Parikh CL, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12, 14-PGJ2 and the ligation of PPAR gamma. J Clin Invest. 2003;112:945–55.
Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res. 2009;669:1–7.
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPAR-gamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–43.
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW Jr, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20.
Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–9.
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82.
Kalajdzic T, Faour WH, He QW, Martel-Pelletier J, Pelletier JP, Di Battista JA. Nimesulide, a preferential cyclooxygenase-2 inhibitor, suppresses peroxisomeproliferator activated receptor induction of cyclooxygenase-2 gene expression in human synovial fibroblasts: evidence for receptor antagonism. Arthritis Rheum. 2002;46:494–506.
Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–9.
Zhai B, Yang H, Mancini A, He Q, Antoniou J, Di Battista JA. Leukotriene B4 BLT receptor signaling regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of Ras/Raf/ERK/p42 AUF1 pathway. J Biol Chem. 2010;285:23568–80.
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61.
Brenneis C, Coste O, Altenrath K, Angioni C, Schmidt H, Schuh CD, Zhang DD, Henke M, Weigert A, Brüne B, Rubin B, Nusing R, Scholich K, Geisslinger G. Anti-inflammatory role of microsomal prostaglandin E synthase-1 in a model of neuroinflammation. J Biol Chem. 2011;286:2331–42.
Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45:159–69.
Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–6.
Huang SK, Peters-Golden M. Eicosanoid lipid mediators in fibrotic lung diseases: ready for prime time? Chest. 2008;133:1442–50.
Herrerias A, Torres R, Serra M, Marco A, Pujols L, Picado C, de Mora F. Activity of the cyclooxygenase 2-prostaglandin-E prostanoid receptor pathway in mice exposed to house dust mite aeroallergens, and impact of exogenous prostaglandin E2. J Inflamm (Lond). 2009;6:30.
Hogaboam CM, Bissonnette EY, Chin BC, Befus AD, Wallace JL. Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterology. 1993;104:122–9.
Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Control by IFN-g and PGE2 of TNFa and IL-1 production by human monocytes. Immunology. 1989;66:376–83.
Takayama K, García-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–54.
Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15:15–21.
Largo R, Díez-Ortego I, Sanchez-Pernaute O, López-Armada MJ, Alvarez-Soria MA, Egido J, Herrero-Beaumont G. EP2/EP4 signalling inhibits monocyte chemoattractant protein-1 production induced by interleukin 1b in synovial fibroblasts. Ann Rheum Dis. 2004;63:1197–204.
Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74:868–79.
Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–32.
Wertheim WA, Kunkel SL, Standiford TJ, Burdick MD, Becker FS, Wilke CA, Gilbert AR, Strieter RM. Regulation of neutrophil-derived IL-8: the role of prostaglandin E2, dexamethasone, and IL-4. J Immunol. 1993;151:2166–75.
Pouliot M, Fiset ME, Massé M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–86.
St-Onge M, Flamand N, Biarc J, Picard S, Bouchard L, Dussault AA, Laflamme C, James MJ, Caughey GE, Cleland LG, Borgeat P, Pouliot M. Characterization of prostaglandin E2 generation through the cyclooxygenase (COX)-2 pathway in human neutrophils. Biochem Biophys Acta. 2007;1771:1235–45.
Persson S, Mikulowska A, Narula S, O’Garra A, Holmdahl R. Interleukin-10 suppresses the development of collagen type II-induced arthritis and ameliorates sustained arthritis in rats. Scand J Immunol. 1996;44:607–14.
Joosten LA, Lubberts E, Durez P, Helsen MM, Jacobs MJ, Goldman M, van den Berg WB. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–60.
Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Britti D, De Maio M, Caputi AP. Absence of endogeneous interleukin-10 enhances the evolution of murine type-II collagen-induced arthritis. Eur Cytokine Network. 2001;12:568–80.
Finnegan A, Kaplan CD, Cao Y, Eibel H, Glant TT, Zhang J. Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther. 2003;5:R18–24.
Evans K, Fox S. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;8:4.
Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a structure with features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–56.
Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Anti-inflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase. J Exp Med. 2006;203:1883–9.
Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30.
Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J Clin Invest. 2003;111:1107–13.
Sugimoto Y, Naruyima S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7.
Gerarduzzi C. PhD Thesis, Department of Experimental Medicine, McGill University, 2009. Characterizing the activating and attenuating signal scaffolding units of the PGE2-EP receptor system in normal and arthritic human synovial fibroblasts (John A and Di Battista, Supervisor).
Faour W, He HY, et al. Prostaglandin E2 regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1β-treated human synovial fibroblasts. J Biol Chem. 2001;276:31720–31.
Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, Park J, Bingham CO 3rd, Al-Mussawir H, Abramson SB. Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol. 2005;175:6924–30.
Min SY, Kim WU, Cho ML, Hwang SY, Park SH, Cho CS, Kim JM, Kim HY. Prostaglandin E2 suppresses nuclear factor-kappaB mediated interleukin 15 production in rheumatoid synoviocytes. J Rheumatol. 2002;29:1366–76.
Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6.
Ott RG, Simma O, Kollmann K, Weisz E, Zebedin EM, Schorpp-Kistner M, Heller G, Zöchbauer S, Wagner EF, Freissmuth M, Sexl V. JunB is a gatekeeper for B lymphoid leukemia. Oncogene. 2007;26:4863–71.
Passegue E, Wagner EF. JunB suppresses cell proliferation by transcriptional activation of p16 (INK4a) expression. EMBO J. 2000;19:2969–79.
Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9.
Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–7.
Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–8.
Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–46.
Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16.
Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74.
Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kühn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8.
Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65.
Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–15.
Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–52.
Clish CB, Levy BD, Chiang N, Tai HH, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation. J Biol Chem. 2000;275:25372–80.
Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal anti-inflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204.
Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37.
Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22.
József L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirintriggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–71.
Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–6.
Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Posttranscriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem. 2000;275:11750–7.
Mancini A, Jovanovic DV, He QW, Di Battista JA. Site-specific proteolysis of cyclooxygenase-2: a putative step in inflammatory prostaglandin E(2) biosynthesis. J Cell Biochem. 2007;101:425–41.
Mancini AD, Di Battista JA. Tristetraprolin: a prostaglandin E2-responsive bifunctional regulator of cyclooxygenase-2 expression. Osteoarthr Cartil. 2008;16(Suppl 4):S16.
Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–23.
Raghavan A, Robison RL, McNabb J, Miller CR, Williams DA, Bohjanen PR. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J Biol Chem. 2001;276:47958–65.
Yu H, Stasinopoulos S, Leedman P, Medcalf RL. Inherent instability of plasminogen activator inhibitor type 2 mRNA is regulated by tristetraprolin. J Biol Chem. 2003;278:13912–8.
Sully G, Dean JL, Wait R, Rawlinson L, Santalucia T, Saklatvala J, Clark AR. Structural and functional dissection of a conserved destabilizing element of cyclooxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1). Biochem J. 2004;377(Pt 3):629–39.
Tchen CR, Brook M, Saklatvala J, Clark AR. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem. 2004;279:32393–400.
Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813–27.
Jalonen U, Nieminen R, Vuolteenaho K, Kankaanranta H, Moilanen E. Downregulation of tristetraprolin expression results in enhanced IL-12 and MIP-2 production and reduced MIP-3 alpha synthesis in activated macrophages. Mediators Inflamm. 2006;2006:40691.
Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36:491–6.
Tegeder I, Niederberger E, Israr E, Gühring H, Brune K, Euchenhofer C, Grösch S, Geisslinger G. Inhibition of NF-kappaB and AP-1 activation by R- and S- flurbiprofen. FASEB J. 2001;15:2–4.
Fries JF. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol. 1991;28:6–10.
Brooks PM, Day RO. Nonsteroidal anti-inflammatory drugs—differences and similarities. New Engl J Med. 1991;324:1716–25.
Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:352–6.
Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202.
Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, Halevy S. Drug exposure and psoriasis vulgaris: case-control and case-crossover studies. Acta Derm Venereol. 2005;85:299–303.
Katayama H, Kawada A. Exacerbation of psoriasis induced by indomethacin. J Dermatol. 1981;8:323–7.
Wang XM, Wu TX, Lee YS, Dionne RA. Rofecoxib regulates the expression of genes related to the matrix metalloproteinase pathway in humans: implication for the adverse effects of cyclooxygenase-2 inhibitors. Clin Pharmacol Ther. 2006;79:303–15.
Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2011;50:35–51.
Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37.
Proceedings of the 9th World Congress on Inflammation, July 6–10 2009, Tokyo, Japan. Inflammation Res. 58(Suppl 2):S79–S279.
Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA. J Biol Chem. 2007;282:3766–77.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Mancini, A.D., Di Battista, J.A. The cardinal role of the phospholipase A2/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E2 (PCPP) axis in inflammostasis. Inflamm. Res. 60, 1083–1092 (2011). https://doi.org/10.1007/s00011-011-0385-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0385-7