Abstract
Background
Inflammation is widely recognized as the driving force of cachexia induced by chronic diseases; however, therapies targeting inflammation do not always reverse cachexia. Thus, whether inflammation per se plays an important role in the clinical course of cachectic patients is still a matter of debate.
Aims
To give new insights into cachexia’s pathogenesis and diagnosis, we performed a comprehensive literature search on the contribution of inflammatory markers to this syndrome, focusing on the noncommunicable diseases cancer and cardiovascular diseases.
Methods
A systematic review was performed in PubMed using the keywords (“cancer” OR “cardiac” cachexia AND “human” OR “patient” AND “plasma” or “serum”). A total of 744 studies were retrieved and, from these, 206 were selected for full-text screening. In the end, 98 papers focusing on circulating biomarkers of cachexia were identified, which resulted in a list of 113 different mediators.
Results
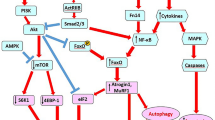
Data collected from the literature highlight the contribution of interleukin-6 (IL-6) and C-reactive protein (CRP) to cachexia, independently of the underlying condition. Despite not being specific, once the diagnosis of cachexia is established, CRP might help to monitor the effectiveness of anti-cachexia therapies. In cardiac diseases, B-type natriuretic peptide (BNP), renin, and obestatin might be putative markers of body wasting, whereas in cancer, growth differentiation factor (GDF) 15, transforming growth factor (TGF)-β1 and vascular endothelial growth factor (VEGF) C seem to be better markers of this syndrome. Independently of the circulating mediators, NF-κB and JAK/STAT signaling pathways play a key role in bridging inflammation with muscle wasting; however, therapies targeting these pathways were not proven effective for all cachectic patients.
Conclusion
The critical and integrative analysis performed herein will certainly feed future research focused on the better comprehension of cachexia pathogenesis toward the improvement of its diagnosis and the development of personalized therapies targeting specific cachexia phenotypes.



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. Biomed Res Int. 2014;2014:168407. https://doi.org/10.1155/2014/168407.
Schcolnik-Cabrera A, Chavez-Blanco A, Dominguez-Gomez G, Duenas-Gonzalez A. Understanding tumor anabolism and patient catabolism in cancer-associated cachexia. Am J Cancer Res. 2017;7:1107–35.
Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–62. https://doi.org/10.1038/nrc3829.
Argiles JM, Betancourt A, Guardia-Olmos J, et al. Validation of the CAchexia SCOre (CASCO) staging cancer patients: the use of miniCASCO as a simplified tool. Front Physiol. 2017;8:92. https://doi.org/10.3389/fphys.2017.00092.
von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323–41. https://doi.org/10.1038/nrcardio.2017.51.
Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–83. https://doi.org/10.1016/S0140-6736(03)12892-9.
Christensen HM, Kistorp C, Schou M, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626–34. https://doi.org/10.1007/s12020-012-9836-3.
von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachex Sarcopenia Muscle. 2010;1:1–5. https://doi.org/10.1007/s13539-010-0002-6.
Saitoh M, Ishida J, Doehner W, et al. Sarcopenia, cachexia, and muscle performance in heart failure: review update 2016. Int J Cardiol. 2017;238:5–11. https://doi.org/10.1016/j.ijcard.2017.03.155.
Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond). 2005;108:205–13. https://doi.org/10.1042/CS20040174.
Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure–the whys and wherefores. Heart Fail Rev. 2006;11:83–92. https://doi.org/10.1007/s10741-006-9196-2.
Martins T, Vitorino R, Moreira-Goncalves D, Amado F, Duarte JA, Ferreira R. Recent insights on the molecular mechanisms and therapeutic approaches for cardiac cachexia. Clin Biochem. 2014;47:8–15. https://doi.org/10.1016/j.clinbiochem.2013.10.025.
Antunes JMM, Ferreira RMP, Moreira-Goncalves D. Exercise training as therapy for cancer-induced cardiac cachexia. Trends Mol Med. 2018. https://doi.org/10.1016/j.molmed.2018.06.002.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. https://doi.org/10.1038/nature21349.
Van Linthout S, Tschöpe C. Inflammation – cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14:251–65. https://doi.org/10.1007/s11897-017-0337-9.
Tisdale MJ. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 2010;6:503–13. https://doi.org/10.2217/fon.10.20.
Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–89. https://doi.org/10.1002/ejhf.942.
Tousoulis D, Charakida M, Stefanadis C. Inflammation and endothelial dysfunction as therapeutic targets in patients with heart failure. Int J Cardiol. 2005;100:347–53. https://doi.org/10.1016/j.ijcard.2004.05.030.
Argiles JM. The 2015 ESPEN Sir David Cuthbertson lecture: inflammation as the driving force of muscle wasting in cancer. Clin Nutr. 2017;36:798–803. https://doi.org/10.1016/j.clnu.2016.05.010.
Klein GL, Petschow BW, Shaw AL, Weaver E. Gut barrier dysfunction and microbial translocation in cancer cachexia: a new therapeutic target. Curr Opin Support Palliat Care. 2013;7:361–7. https://doi.org/10.1097/SPC.0000000000000017.
Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–35. https://doi.org/10.1096/fj.11-197921.
Genton L, Mareschal J, Charretier Y, Lazarevic V, Bindels LB, Schrenzel J. Targeting the gut microbiota to treat cachexia. Front Cell Infect Microbiol. 2019. https://doi.org/10.3389/fcimb.2019.00305.
Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200–e200. https://doi.org/10.1038/oncsis.2016.3.
Pajak B, Orzechowska S, Pijet B, Pijet M, Pogorzelska A, Gajkowska B, Orzechowski A. Crossroads of cytokine signaling–the chase to stop muscle cachexia. J Physiol Pharmacol. 2008;59(Suppl 9):251–64.
Blaes A, Prizment A, Koene RJ, Konety S. Cardio-oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin. 2017;13:367–80. https://doi.org/10.1016/j.hfc.2016.12.006.
Bolat I, Biteker M. Modified glasgow prognostic score is a novel predictor of clinical outcome in heart failure with preserved ejection fraction. Scand Cardiovasc J. 2020. https://doi.org/10.1080/14017431.2019.1709656.
Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–97. https://doi.org/10.1016/s0161-5890(01)00042-6.
Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–11. https://doi.org/10.1016/j.clim.2005.08.004.
Schimmack S, Yang Y, Felix K, et al. C-reactive protein (CRP) promotes malignant properties in pancreatic neuroendocrine neoplasms. Endocr Connect. 2019;8:1007–19. https://doi.org/10.1530/EC-19-0132.
Verma S, Szmitko PE, Yeh ET. C-reactive protein: structure affects function. Circulation. 2004;109:1914–7. https://doi.org/10.1161/01.CIR.0000127085.32999.64.
Kitagawa M, Haji S, Amagai T. High serum essential amino acids as a predictor of skeletal muscle depletion in patients with cachexia and advanced gastrointestinal cancers. Nutr Clin Pract. 2017;32:645–51. https://doi.org/10.1177/0884533617724742.
Tavares P, Gonçalves DM, Santos LL, Ferreira R. Revisiting the clinical usefulness of C-reactive protein in the set of cancer cachexia. Porto Biomed J. 2021;6:e123. https://doi.org/10.1097/j.pbj.0000000000000123.
Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33:737–48. https://doi.org/10.1016/j.clnu.2014.03.007.
Patel HJ, Patel BM. TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017;170:56–63. https://doi.org/10.1016/j.lfs.2016.11.033.
Weitzel LB, Ambardekar AV, Brieke A, Cleveland JC, Serkova NJ, Wischmeyer PE, Lowes BD. Left ventricular assist device effects on metabolic substrates in the failing heart. PLoS ONE. 2013;8:e60292. https://doi.org/10.1371/journal.pone.0060292.
Miller J, Alshehri A, Ramage MI, et al. Plasma metabolomics identifies lipid and amino acid markers of weight loss in patients with upper gastrointestinal cancer. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11101594.
Hou YC, Wang CJ, Chao YJ, et al. Elevated serum interleukin-8 level correlates with cancer-related cachexia and sarcopenia: an indicator for pancreatic cancer outcomes. J Clin Med. 2018. https://doi.org/10.3390/jcm7120502.
Fujiwara Y, Kobayashi T, Chayahara N, et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS ONE. 2014;9:e113259. https://doi.org/10.1371/journal.pone.0113259.
Gulen ST, Karadag F, Karul AB, Kilicarslan N, Ceylan E, Kuman NK, Cildag O. Adipokines and systemic inflammation in weight-losing lung cancer patients. Lung. 2012;190:327–32. https://doi.org/10.1007/s00408-011-9364-6.
Agustsson T, Ryden M, Hoffstedt J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–7. https://doi.org/10.1158/0008-5472.CAN-06-4585.
Refsgaard Holm M, Christensen H, Rasmussen J, Johansen ML, Schou M, Faber J, Kistorp C. Fibroblast growth factor 21 in patients with cardiac cachexia: a possible role of chronic inflammation. ESC Heart Fail. 2019;6:983–91. https://doi.org/10.1002/ehf2.12502.
la Cour JL, Christensen HM, Köhrle J, Lehmphul I, Kistorp C, Nygaard B, Faber J. Association between 3-Iodothyronamine (T1am) concentrations and left ventricular function in chronic heart failure. J Clin Endocrinol Metab. 2018;104:1232–8. https://doi.org/10.1210/jc.2018-01466.
Christensen HM, Kistorp C, Schou M, et al. Cross-talk between the heart and adipose tissue in cachectic heart failure patients with respect to alterations in body composition: a prospective study. Metab Clin Exp. 2014;63:141–9. https://doi.org/10.1016/j.metabol.2013.09.017.
McEntegart MB, Awede B, Petrie MC, Sattar N, Dunn FG, MacFarlane NG, McMurray JJ. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829–35. https://doi.org/10.1093/eurheartj/ehm033.
Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS ONE. 2015;10:e0143080–e0143080. https://doi.org/10.1371/journal.pone.0143080.
Lavriv DS, Neves PM, Ravasco P. Should omega-3 fatty acids be used for adjuvant treatment of cancer cachexia? Clin Nutr ESPEN. 2018;25:18–25. https://doi.org/10.1016/j.clnesp.2018.02.006.
Maddocks M, Jones LW, Wilcock A. Immunological and hormonal effects of exercise: implications for cancer cachexia. Curr Opin Support Palliat Care. 2013;7:376–82. https://doi.org/10.1097/SPC.0000000000000010.
Macciò A, Madeddu C, Gramignano G, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012;124:417–25. https://doi.org/10.1016/j.ygyno.2011.12.435.
Gray S, Axelsson B. The prevalence of deranged C-reactive protein and albumin in patients with incurable cancer approaching death. PLoS ONE. 2018;13:e0193693. https://doi.org/10.1371/journal.pone.0193693.
Lerner L, Tao J, Liu Q, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7:467–82. https://doi.org/10.1002/jcsm.12077.
Bindels LB, Neyrinck AM, Loumaye A, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9:18224–38. https://doi.org/10.18632/oncotarget.24804.
Kayacan O, Karnak D, Beder S, Gullu E, Tutkak H, Senler FC, Koksal D. Impact of TNF-alpha and IL-6 levels on development of cachexia in newly diagnosed NSCLC patients. Am J Clin Oncol. 2006;29:328–35. https://doi.org/10.1097/01.coc.0000221300.72657.e0.
Weber MA, Kinscherf R, Krakowski-Roosen H, et al. Myoglobin plasma level related to muscle mass and fiber composition: a clinical marker of muscle wasting? J Mol Med (Berl). 2007;85:887–96. https://doi.org/10.1007/s00109-007-0220-3.
Yang QJ, Zhao JR, Hao J, et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9:71–85. https://doi.org/10.1002/jcsm.12246.
Moses AGW, Maingay J, Sangster K, Fearon KCH, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009;21:1091–5. https://doi.org/10.3892/or_00000328.
Witte KKA, Ford SJ, Preston T, Parker JD, Clark AL. Fibrinogen synthesis is increased in cachectic patients with chronic heart failure. Int J Cardiol. 2008;129:363–7. https://doi.org/10.1016/j.ijcard.2007.07.119.
Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288–94. https://doi.org/10.1158/2326-6066.CIR-14-0022.
Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41. https://doi.org/10.1016/j.semcdb.2016.02.009.
Bonetto A, Aydogdu T, Jin X, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410-421. https://doi.org/10.1152/ajpendo.00039.2012.
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. https://doi.org/10.1038/nrendo.2012.49.
Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–48. https://doi.org/10.1111/febs.12338.
Garcia JM, Garcia-Touza M, Hijazi RA, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–6. https://doi.org/10.1210/jc.2004-1788.
Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700-709. https://doi.org/10.1210/jc.2011-2387.
de Castro GS, Correia-Lima J, Simoes E, et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin Nutr. 2021;40:2443–55. https://doi.org/10.1016/j.clnu.2020.10.050.
Bilir C, Engin H, Can M, Temi YB, Demirtas D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol. 2015;32:56. https://doi.org/10.1007/s12032-015-0497-y.
Fogelman DR, Morris J, Xiao L, et al. A predictive model of inflammatory markers and patient-reported symptoms for cachexia in newly diagnosed pancreatic cancer patients. Support Care Cancer. 2017;25:1809–17. https://doi.org/10.1007/s00520-016-3553-z.
Riccardi D, das Neves RX, de Matos-Neto EM, et al. Plasma lipid profile and systemic inflammation in patients with cancer cachexia. Front Nutr. 2020;7:4. https://doi.org/10.3389/fnut.2020.00004.
Kemik O, Sumer A, Kemik AS, et al. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol. 2010;8:85–85. https://doi.org/10.1186/1477-7819-8-85.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Banas T. Impact of weight loss on circulating IL-1, IL-6, IL-8, TNF-alpha, VEGF-A, VEGF-C and midkine in gastroesophageal cancer patients. Clin Biochem. 2007;40:1353–60. https://doi.org/10.1016/j.clinbiochem.2007.07.013.
Tazaki E, Shimizu N, Tanaka R, et al. Serum cytokine profiles in patients with prostate carcinoma. Exp Ther Med. 2011;2:887–91. https://doi.org/10.3892/etm.2011.286.
Dulger H, Alici S, Sekeroglu MR, Erkog R, Ozbek H, Noyan T, Yavuz M. Serum levels of leptin and proinflammatory cytokines in patients with gastrointestinal cancer. Int J Clin Pract. 2004;58:545–9. https://doi.org/10.1111/j.1368-5031.2004.00149.x.
Richey LM, George JR, Couch ME, et al. Defining cancer cachexia in head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:6561–7. https://doi.org/10.1158/1078-0432.CCR-07-0116.
Filippatos GS, Tsilias K, Venetsanou K, et al. Leptin serum levels in cachectic heart failure patients. Relationship with tumor necrosis factor-alpha system. Int J Cardiol. 2000;76:117–22. https://doi.org/10.1016/s0167-5273(00)00397-1.
Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–93. https://doi.org/10.1053/euhj.1998.1446.
Martinez-Hernandez PL, Hernanz-Macias A, Gomez-Candela C, et al. Serum interleukin-15 levels in cancer patients with cachexia. Oncol Rep. 2012;28:1443–52. https://doi.org/10.3892/or.2012.1928.
Lerner L, Hayes TG, Tao N, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:317–24. https://doi.org/10.1002/jcsm.12033.
Fortunati N, Manti R, Birocco N, et al. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep. 2007;18:1521–7.
Shibata M, Takekawa M, Amano S. Increased serum concentrations of soluble tumor necrosis factor receptor I in noncachectic and cachectic patients with advanced gastric and colorectal cancer. Surg Today. 1998;28:884–8. https://doi.org/10.1007/s005950050247.
Takahashi M, Terashima M, Takagane A, Oyama K, Fujiwara H, Wakabayashi G. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int J Clin Oncol. 2009;14:315–20. https://doi.org/10.1007/s10147-008-0856-1.
Batista ML, Olivan M, Alcantara PSM, et al. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine. 2013;61:532–9. https://doi.org/10.1016/j.cyto.2012.10.023.
Shibata M, Nezu T, Takekawa M, et al. Serum levels of interleukin-10 and interleukin-12 in patients with colorectal cancer. Ann N Y Acad Sci. 1996;795:410–2. https://doi.org/10.1111/j.1749-6632.1996.tb52707.x.
Zhang H, Xu X. Mutation-promoting molecular networks of uncontrolled inflammation. Tumour Biol. 2017;39:1010428317701310. https://doi.org/10.1177/1010428317701310.
Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol. 2004;1:343–50.
Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–43. https://doi.org/10.1093/ajcn/83.4.735.
Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–31. https://doi.org/10.1016/j.febslet.2007.11.051.
Han HQ, Mitch WE. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr Opin Support Palliat Care. 2011;5:334–41. https://doi.org/10.1097/SPC.0b013e32834bddf9.
Moreira-Goncalves D, Padrao AI, Ferreira R, et al. Signaling pathways underlying skeletal muscle wasting in experimental pulmonary arterial hypertension. Biochim Biophys Acta. 2015;1852:2722–31. https://doi.org/10.1016/j.bbadis.2015.10.002.
Yoshida T, Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350:250–6. https://doi.org/10.1097/MAJ.0000000000000511.
Padrao AI, Oliveira P, Vitorino R, et al. Bladder cancer-induced skeletal muscle wasting: disclosing the role of mitochondria plasticity. Int J Biochem Cell Biol. 2013;45:1399–409. https://doi.org/10.1016/j.biocel.2013.04.014.
Loumaye A, de Barsy M, Nachit M, et al. Role of activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100:2030–8. https://doi.org/10.1210/jc.2014-4318.
Tisdale MJ. The “cancer cachectic factor.” Support Care Cancer. 2003;11:73–8. https://doi.org/10.1007/s00520-002-0408-6.
Penafuerte CA, Gagnon B, Sirois J, Murphy J, MacDonald N, Tremblay ML. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer. 2016;114:680–7. https://doi.org/10.1038/bjc.2016.3.
McCartney-Francis N, Jin W, Wahl SM. Aberrant toll receptor expression and endotoxin hypersensitivity in mice lacking a functional TGF-beta 1 signaling pathway. J Immunol. 2004;172:3814–21. https://doi.org/10.4049/jimmunol.172.6.3814.
Waning DL, Mohammad KS, Reiken S, et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21:1262–71. https://doi.org/10.1038/nm.3961.
Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–40. https://doi.org/10.1038/nm1677.
Ahmed DS, Isnard S, Lin J, Routy B, Routy J-P. GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J Cancer. 2021;12:1125–32. https://doi.org/10.7150/jca.50376.
Ost M, Igual Gil C, Coleman V, et al. Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress. EMBO Rep. 2020;21:e48804–e48804. https://doi.org/10.15252/embr.201948804.
Lai Y-C, Provencher S, Goncharova EA. TAKling GDF-15 and skeletal muscle atrophy in pulmonary hypertension: are we there yet? Thorax. 2019;74:103–5. https://doi.org/10.1136/thoraxjnl-2018-212680.
Laurens C, Parmar A, Murphy E, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020;5:e131870. https://doi.org/10.1172/jci.insight.131870.
Bloch SAA, Lee JY, Wort SJ, Polkey MI, Kemp PR, Griffiths MJD. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery*. Crit Care Med. 2013. https://doi.org/10.1097/CCM.0b013e318274671b.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. https://doi.org/10.1038/nrm1911.
Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, et al. Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med. 2008;46:359–64. https://doi.org/10.1515/CCLM.2008.089.
Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–65. https://doi.org/10.1016/j.ceb.2008.12.012.
Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–56. https://doi.org/10.1038/nrc2524.
Rauniyar K, Jha SK, Jeltsch M. Biology of vascular endothelial growth factor C in the morphogenesis of lymphatic vessels. Front Bioeng Biotechnol. 2018;6:7. https://doi.org/10.3389/fbioe.2018.00007.
Zhao T, Zhao W, Meng W, Liu C, Chen Y, Sun Y. Vascular endothelial growth factor-C: its unrevealed role in fibrogenesis. Am J Physiol Heart Circ Physiol. 2014;306:H789-796. https://doi.org/10.1152/ajpheart.00559.2013.
Smythe G. Role of growth factors in modulation of the microvasculature in adult skeletal muscle. Adv Exp Med Biol. 2016;900:161–83. https://doi.org/10.1007/978-3-319-27511-6_7.
Lacquaniti A, Donato V, Chirico V, Buemi A, Buemi M. Obestatin: an interesting but controversial gut hormone. Ann Nutr Metab. 2011;59:193–9. https://doi.org/10.1159/000334106.
Xin X, Ren AJ, Zheng X, Qin YW, Zhao XX, Yuan WJ, Guo ZF. Disturbance of circulating ghrelin and obestatin in chronic heart failure patients especially in those with cachexia. Peptides. 2009;30:2281–5. https://doi.org/10.1016/j.peptides.2009.07.026.
Huang Q, Fan YZ, Ge BJ, Zhu Q, Tu ZY. Circulating ghrelin in patients with gastric or colorectal cancer. Dig Dis Sci. 2007;52:803–9. https://doi.org/10.1007/s10620-006-9508-3.
Lilleness BM, Frishman WH. Ghrelin and the cardiovascular system. Cardiol Rev. 2016;24:288–97. https://doi.org/10.1097/CRD.0000000000000113.
Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–8. https://doi.org/10.1161/hc4201.097836.
Shen C, Zhou J, Wang X, et al. Angiotensin-II-induced muscle wasting is mediated by 25-hydroxycholesterol via GSK3beta signaling pathway. EBioMedicine. 2017;16:238–50. https://doi.org/10.1016/j.ebiom.2017.01.040.
Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34. https://doi.org/10.1161/01.cir.96.2.526.
Anker SD, Volterrani M, Pflaum CD, et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–52. https://doi.org/10.1016/s0735-1097(01)01385-7.
Kerem M, Ferahkose Z, Yilmaz UT, et al. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol. 2008;14:3633–41. https://doi.org/10.3748/wjg.14.3633.
Huang X-y, Huang Z-l, Yang J-h, et al. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J Exp Clin Cancer Res. 2016;35:46. https://doi.org/10.1186/s13046-016-0317-z.
Trobec K, von Haehling S, Anker SD, Lainscak M. Growth hormone, insulin-like growth factor 1, and insulin signaling-a pharmacological target in body wasting and cachexia. J Cachexia Sarcopenia Muscle. 2011;2:191–200. https://doi.org/10.1007/s13539-011-0043-5.
Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893–8.
Bozcali E, Polat V, Akbulut H, Ferzeyn Yavuzkir M, Karaca I. Serum adiponectin, anemia and left ventricular dimensions in patients with cardiac cachexia. Cardiology. 2013;126:207–13. https://doi.org/10.1159/000353291.
Tedeschi S, Pilotti E, Parenti E, et al. Serum adipokine zinc alpha2-glycoprotein and lipolysis in cachectic and noncachectic heart failure patients: relationship with neurohormonal and inflammatory biomarkers. Metabolism. 2012;61:37–42. https://doi.org/10.1016/j.metabol.2011.05.011.
Paulo Araújo J, Lourenço P, Rocha-Gonçalves F, Ferreira A, Bettencourt P. Adiponectin is increased in cardiac cachexia irrespective of body mass index. Eur J Heart Fail. 2009;11:567–72. https://doi.org/10.1093/eurjhf/hfp046.
Vaz Pérez A, Doehner W, von Haehling S, et al. The relationship between tumor necrosis factor-α, brain natriuretic peptide and atrial natriuretic peptide in patients with chronic heart failure. Int J Cardiol. 2010;141:39–43. https://doi.org/10.1016/j.ijcard.2008.11.146.
Kalkan AK, Cakmak HA, Erturk M, et al. Adropin and irisin in patients with cardiac cachexia. Arq Bras Cardiol. 2018;111:39–47. https://doi.org/10.5935/abc.20180109.
Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther. 2004;102:223–41. https://doi.org/10.1016/j.pharmthera.2004.04.004.
Prado BL, Qian Y. Anti-cytokines in the treatment of cancer cachexia. Ann Palliat Med. 2018;8:67–79. https://doi.org/10.21037/apm.2018.07.06.
Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12:14–6. https://doi.org/10.1002/jcsm.12675.
Gordon JN, Goggin PM. Thalidomide and its derivatives: emerging from the wilderness. Postgrad Med J. 2003;79:127–32. https://doi.org/10.1136/pmj.79.929.127.
Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia – can findings from animal models be translated to humans? BMC Cancer. 2016;16:75. https://doi.org/10.1186/s12885-016-2121-8.
Saccani A, Schioppa T, Porta C, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–40. https://doi.org/10.1158/0008-5472.CAN-06-1867.
Batista ML Jr, Peres SB, McDonald ME, et al. Adipose tissue inflammation and cancer cachexia: possible role of nuclear transcription factors. Cytokine. 2012;57:9–16. https://doi.org/10.1016/j.cyto.2011.10.008.
Penna F, Bonetto A, Aversa Z, Minero VG, Rossi Fanelli F, Costelli P, Muscaritoli M. Effect of the specific proteasome inhibitor bortezomib on cancer-related muscle wasting. J Cachexia Sarcopenia Muscle. 2016;7:345–54. https://doi.org/10.1002/jcsm.12050.
Bossola M, Pacelli F, Tortorelli A, Rosa F, Doglietto GB. Skeletal muscle in cancer cachexia: the ideal target of drug therapy. Curr Cancer Drug Targets. 2008;8:285–98. https://doi.org/10.2174/156800908784533463.
Jatoi A, Dakhil SR, Nguyen PL, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome. Cancer. 2007;110:1396–403. https://doi.org/10.1002/cncr.22944.
Wiedenmann B, Malfertheiner P, Friess H, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18–25.
Jatoi A, Ritter HL, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer. 2010;68:234–9. https://doi.org/10.1016/j.lungcan.2009.06.020.
Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz H-J. Molecular pathways: cachexia signaling-a targeted approach to cancer treatment. Clin Cancer Res. 2016;22:3999–4004. https://doi.org/10.1158/1078-0432.ccr-16-0495.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primer. 2018;4:17105. https://doi.org/10.1038/nrdp.2017.105.
Argilés JM, López-Soriano FJ, Stemmler B, Busquets S. Therapeutic strategies against cancer cachexia. Eur J Transl Myol. 2019;29:7960–7960. https://doi.org/10.4081/ejtm.2019.7960.
Okoshi MP, Capalbo RV, Romeiro FG, Okoshi K. Cardiac cachexia: perspectives for prevention and treatment. Arq Bras Cardiol. 2017;108:74–80. https://doi.org/10.5935/abc.20160142.
Del Fabbro E. Combination therapy in cachexia. Ann Palliat Med. 2018;8:59–66. https://doi.org/10.20137/apm.2018.08.05.
Berardi E, Madaro L, Lozanoska-Ochser B, Adamo S, Thorrez L, Bouche M, Coletti D. A pound of flesh: what cachexia is and what it is not. Diagnostics (Basel). 2021;11:116. https://doi.org/10.3390/diagnostics11010116.
Acknowledgements
This work was supported by “Fundação para a Ciência e a Tecnologia”—FCT, European Union, QREN, FEDER and COMPETE for funding the LAQV-REQUIMTE (UIDB/50006/2020), UnIC (UIDB/IC/00051/2020 and UIDP/00051/2020), and CIAFEL (UIDB/00617/2020) research units, RISE – Health Research Network-From the Lab to the Community (LA/P/0053/2020), ITR—Laboratory for Integrative and Translational Research in Population Health (LA/P/0064/2020) and the research projects DOCnet (NORTE-01-0145-FEDER-000003), NETDIAMOND (SAICT-PAC/0047/2015), and PROTECT (PTDC/SAU-DES/7945/2020). Rita Nogueira-Ferreira acknowledges FCT for the research contract CEECIND/03935/2021 under the CEEC Individual 2021.
Author information
Authors and Affiliations
Contributions
Authors RNF, FSN, and LMS: performed the literature search and wrote the manuscript. RNF and RF: conceptualized the review. RF and DMG: wrote the first draft and edited the review. ALM, RV, and LLS: critically revised the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors contributed to the article and approved the submitted version.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nogueira-Ferreira, R., Sousa-Nunes, F., Leite-Moreira, A. et al. Cancer- and cardiac-induced cachexia: same fate through different inflammatory mediators?. Inflamm. Res. 71, 771–783 (2022). https://doi.org/10.1007/s00011-022-01586-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01586-y