Abstract
Objective
Traumatic brain injury (TBI) is a significant cause of death and long-term deficits in motor and cognitive functions for which there are currently no effective chemotherapeutic drugs. Bazedoxifene (BZA) is a third-generation selective estrogen receptor modulator (SERM) and has been investigated as a treatment for postmenopausal osteoporosis. It is generally safe and well tolerated, with favorable endometrial and breast safety profiles. Recent findings have shown that SERMs may have therapeutic benefits; however, the role of BZA in the treatment of TBI and its molecular and cellular mechanisms remain poorly understood. The aim of the present study was to examine the neuroprotective effects of BZA on early TBI in rats and to explore the underlying mechanisms of these effects.
Materials and methods
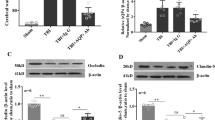
TBI was induced using a modified weight-drop method. Neurological deficits were evaluated according to the neurological severity score (NSS). Morris water maze and open-field behavioral tests were used to test cognitive functions. Brain edema was measured by brain water content, and impairments in the blood–brain barrier (BBB) were evaluated by expression analysis of tight junction-associated proteins, such as occludin and zonula occludens-1 (ZO-1). Neuronal injury was assessed by hematoxylin and eosin (H&E) staining. LC–MS/MS analysis was performed to determine the ability of BZA to cross the BBB.
Results
Our results indicated that BZA attenuated the impaired cognitive functions and the increased BBB permeability of rats subjected to TBI through activation of inflammatory cascades. In vivo experiments further revealed that BZA provided this neuroprotection by suppressing TBI-induced activation of the MAPK/NF-κB signaling pathway. Thus, mechanically, the anti-inflammatory effects of BZA in TBI may be partially mediated by blocking the MAPK signaling pathway.
Conclusions
These findings suggest that BZA might attenuate neurological deficits and BBB damage to protect against TBI by blocking the MAPK/NF-κB signaling pathway.






Similar content being viewed by others
References
Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier break down as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403.
Li S, Zaninotto AL, Neville IS, Paiva WS, Nunn D, Fregni F. Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence. Neuropsychiatr Dis Treat. 2015;11:1573–86.
Mustafa AG, Alshboul OA. Pathophysiology of traumatic brain injury. Neurosciences (Riyadh). 2013;18:222–34.
Moretti R, Pansiot J, Bettati D, Strazielle N, Ghersi-Egea JF, Damante G, Fleiss B, Titomanlio L, Gressens P. Blood–brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 2015;9:40.
Schwarzmaier SM, Plesnila N. Contributions of the immunesystem to the pathophysiology of traumatic brain injury-evidence by intravital microscopy. Front Cell Neurosci. 2014;8:358.
Li H, Sun J, Wang F, Ding G, Chen W, Fang R, Yao Y, Pang M, Lu ZQ, Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood–brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–8.
Blixt J, Svensson M, Gunnarson E, Wanecek M. Aquaporins and blood–brain barrier permeability in early edema development after traumatic brain injury. Brain Res. 2015;1611:18–28.
Shi H, Wang HL, Pu HJ, Shi YJ, Zhang J, Zhang WT, Wang GH, Hu XM, Leak RK, Chen J, Gao YQ. Ethyl pyruvate protects against blood–brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury. CNS Neurosci Ther. 2015;21:374–84.
Wen J, Qian S, Yang Q, Deng L, Mo Y, Yu Y. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp Ther Med. 2014;8:881–6.
Alves JL. Blood–brain barrier and traumatic brain injury. J Neurosci Res. 2014;92:141–7.
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–31.
Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–7.
Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 2013;125:897–908.
Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57.
Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–16.
Rahman A, Fazal F. Blocking NF-κB: an inflammatory issue. Proc Am Thorac Soc. 2011;8:497–503.
Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26.
Nadler Y, Alexandrovich A, Grigoriadis N, Hartmann T, Rao KS, Shohami E, Stein R. Increased expression of the gamma-secretase components presenilin-1 and nicastrin in activated astrocytes and microglia following traumatic brain injury. Glia. 2008;56:552–67.
Zhang ZY, Zhang Z, Fauser U, Schluesener HJ. Global hypomethylation defines a sub-population of reactive microglia/macrophages in experimental traumatic brain injury. Neurosci Lett. 2007;429:1–6.
Ralay Ranaivo H, Wainwright MS. Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res. 2010;1313:222–31.
Xie N, Wang C, Lin Y, Li H, Chen L, Zhang T, Sun Y, Zhang Y, Yin D, Chi Z. The role of p38 MAPK in valproic acid induced microglia apoptosis. Neurosci Lett. 2010;482:51–6.
Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, Kim SH, Kim JG, Kim CH. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–81.
Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163–81.
Gatti D, Rossini M, Sblendorio I, Lello S. Pharmacokinetic evaluation of bazedoxifene for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2013;9:883–92.
Palacios S. Third generation SERMs: anything new? Maturitas. 2010;67:101–2.
Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008.
Komm BS, Lyttle CR. Developing a SERM: stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci. 2001;949:317–26.
Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–51.
Baskin YK1, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93.
García-Rivera D, Delgado R, Bougarne N, Haegeman G, Berghe WV. Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cells. Cancer Lett. 2011;305:21–31.
Kim HN, Kim DH, Kim EH, Lee MH, Kundu JK, Na HK, Cha YN, Surh YJ. Sulforaphane inhibits phorbol ester-stimulated IKK-NF-κB signaling and COX-2 expression inhuman mammary epithelial cells by targeting NF-κB activating kinase and ERK. Cancer Lett. 2014;351:41–9.
Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–49.
Diaz-Arrastia R, Kochanek PM, Bergold P, Kenney K, Marx CE, Grimes CJ, Loh LT, Adam LT, Oskvig D, Curley KC, Salzer W. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J Neurotrauma. 2014;31:135–58.
Kokiko ON, Murashov AK, Hoane MR. Administration of raloxifene reduces sensorimotor and working memory deficits following traumatic brain injury. Behav Brain Res. 2006;170:233–40.
Raghava N, Das BC, Ray SK. Neuroprotective effects of estrogen in CNS injuries: insights from animal models. Neurosci Neuroecon. 2017;6:15–29.
Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res Bull. 2014;109:22–31.
Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–9.
Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J Neurotrauma. 2005;22:345–52.
Lim SW, Nyam Tt E, Hu CY, Chio CC, Wang CC, Kuo JR. Estrogen receptor-α is involved in tamoxifen neuroprotective effects in a traumatic brain injury male rat model. World Neurosurg. 2018;112:e278–87.
Tsai YT, Wang CC, Leung PO, Lin KC, Chio CC, Hu CY, Kuo JR. Extracellular signal-regulated kinase 1/2 is involved in a tamoxifen neuroprotective effect in a lateral fluid percussion injury rat model. J Surg Res. 2014;189:106–16.
Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, Yuen CK, Levine AB, Chines AA, Constantine GD. Constantine, Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause. 2009;16:1109–15.
Kanis JA, Johansson H, Oden A, McCloskey EV. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone. 2009;44:1049–54.
Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516.
Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D 3rd, Li X, Guthikonda M, Rossi NF, Ding Y. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood–brain barrier disruption and braine dema aftertraumatic brain injury. J Neurosurg. 2011;114:92–101.
Zhao YZ, Zhang M, Liu HF1. Wang JP1. Progesterone is neuroprotective by inhibiting cerebral edema after ischemia. Neural Regen Res. 2015;10:1076–81.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405.
Chen T, Liu W, Chao X, Qu Y, Zhang L, Luo P, Xie K, Huo J, Fei Z. Neuroprotective effect of osthole against oxygen and glucose deprivation in rat cortical neurons: involvement of mitogen-activated protein kinase pathway. Neuroscience. 2011;183:203–11.
Chen T, Cao L, Dong W, Luo P, Liu W, Qu Y, Fei Z. Protective effects of mGluR5 positive modulators against traumatic neuronal injury through PKC-dependent activation of MEK/ERK pathway. Neurochem Res. 2012;37:983–90.
Drean A, Goldwirt L, Verreault M. Blood–brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev Neurother. 2016;16:1285–300.
Lin JH. CSF as a surrogate for assessing CNS exposure: an industrial perspective. Curr Drug Metab. 2008;9:46–59.
Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil. 2013;94:1199–201.
Colantonio A. Sex. Gender, and traumatic brain injury: a commentary. Arch Phys Med Rehabil. 2016;97:1–4.
Saverino C, Swaine B, Jaglal S, Lewko J, Vernich L, Voth J, Calzavara A, Colantonio A. Rehospitalization after traumatic brain injury: a population-based study. Arch Phys Med Rehabil. 2016;97:19–25.
Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–26.
Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav Brain Res. 2007;181:200–9.
Acknowledgements
This work is supported by grants from National Natural Science Foundation of China (nos. 81372714, 81672480), Liaoning Provincial Natural Science Foundation of China (no. 201602244), Distinguished Professor Project of Liaoning Province, Special Grant for Translational Medicine, Dalian Medical University (no. 2015002), Basic research projects in colleges and universities of Liaoning Province (no. LQ2017033).
Funding
This work is supported by grants from National Natural Science Foundation of China (nos. 81372714, 81672480), Liaoning Provincial Natural Science Foundation of China (no. 201602244), Distinguished Professor Project of Liaoning Province, Special Grant for Translational Medicine, Dalian Medical University (no. 2015002), Basic research projects in colleges and universities of Liaoning Province (no. LQ2017033).
Author information
Authors and Affiliations
Contributions
Conceptualization, YLL; Funding acquisition, BZ; Investigation, YLL, XW, YJZ, JSX, JCL, and SZ; Methodology, YLL and XW; Writing-original draft, YLL; Writing-review and editing, YLL, XW, JCLL, JSX, SZ, BBM, YD, and BZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Responsible Editor: Thiago Mattar Cunha.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lan, YL., Wang, X., Zou, YJ. et al. Bazedoxifene protects cerebral autoregulation after traumatic brain injury and attenuates impairments in blood–brain barrier damage: involvement of anti-inflammatory pathways by blocking MAPK signaling. Inflamm. Res. 68, 311–323 (2019). https://doi.org/10.1007/s00011-019-01217-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01217-z