Abstract
Purposeof Review
This narrative review explored the role of ketogenic diets (KDs) in improving fertility outcomes, low-grade inflammation, body weight, visceral adipose tissue, and its potential use in certain types of cancer, through its favorable actions on mitochondrial function, reactive oxygen species generation, chronic inflammation, and tumor growth inhibition.
Recent Findings
Nutrition is crucial to maintain the female reproductive system’s health. Evidence on the association between diet and female reproductive system has greatly expanded over the last decade, leading to the identification of specific diet therapy, particularly KDs. KDs has been proved to be an effective weight-loss tool. To date, KDs is being increasingly used in the treatment of many diseases, such as obesity, type 2 diabetes mellitus. KDs is a dietary intervention capable of ameliorating the inflammatory state and oxidative stress through several mechanisms.
Summary
Due to the increasing use of KDs beyond obesity, this literature review will provide the latest scientific evidence of its possible use in common disorders of the female endocrine-reproductive tract, and a practical guide to its use in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is responsible for several disorders affecting the female reproductive tract, many of which lead to infertility, such as polycystic ovary syndrome (PCOS) and endometriosis [1,2,3]. In particular, obesity displays a negative effect on women’s reproductive health, predisposing the development or exacerbation of endocrine disorders of the reproductive tract [2, 4]. Visceral adipose tissue expansion, hyperinsulinemia, and low-grade chronic inflammation resulting from obesity, contribute to the harmful and detrimental dysregulation of reproduction and the subsequent development of fertility disturbances [2]. In this regard, both PCOS and endometriosis share a chronic inflammatory status, which is fueled in the presence of obesity [1, 3, 5]. In addition, chronic inflammation cooperates with insulin resistance and hyperandrogenism to constitute an interactive continuum acting on the pathophysiology of PCOS [3].
Moreover, certain female reproductive tract disorders are more common in women with obesity, such as breast and endometrial cancer: one of the links between obesity and female cancers involves, for instance, impaired glucose metabolism, insulin resistance, and hyperinsulinism [6,7,8]. Obesity has been showed to increase the risk of developing breast cancer, especially for the molecular subtype triple negative breast cancer, and to worsen the prognosis [9]. Of note, breast cancer diagnosed before the menopause shows a more aggressive phenotype, and there is consistent interest in promoting prevention dietary strategies in order to reduce the incidence of this neoplasia in premenopause [10].
Finally, menopause, the physiological cessation of reproductive capacity in a woman’s life, tends to be associated with an increased risk of obesity and a shift toward a distribution of abdominal fat with a relative increase in health risks [11, 12]. Changes in body composition during menopause may be caused by a decrease in circulating estrogens, and the relative increase in androgen-to-estrogens ratio, related to altered fat distribution [11, 12]. Weight gain and increased visceral fat distribution associated with menopausal transition, are responsible for secretion of pro-inflammatory adipocytokines, which leads to type 2 diabetes (T2DM) and cardiovascular diseases (CVD) [13, 14]. In this context, dietary strategies to prevent obesity and obesity-related diseases in menopausal women, including sleep disturbances that represent one of the main symptoms of menopause, should be one of the main objectives for Nutritionists [13, 15].
It is clear that in main endocrine disorders of the female reproductive tract, treatment of excess weight is imperative. Optimal management of obesity requires a multidisciplinary approach to promote weight loss, which can reduce its detrimental health effects [16]. Currently, several approaches to weight loss are available, including different dietary treatments, cognitive behavioral interventions, pharmacological therapies, and surgical interventions [17, 18]. Despite this range of weight loss strategies, less than 20% of individuals who try to lose weight are able to achieve and maintain a 10% reduction over a year, with the majority gaining it back within 3–5 years [19]. As individuals regain weight, many of the associated comorbidities have the potential to return, leading to a great deal of frustration and feelings of hopelessness.
According to Trimboli, in recent years there has been confusion in relation to the nomenclature of ketogenic diets (KDs) [20]. The authors clarify that very low-calorie ketogenic diet (VLCKD) is a KD with a very low-calorie content (< 800 kcal), with 30–50 g of carbohydrates, normoproteic (1.2–1.5 g pro kg), hypolipidic (about 20–40 g/die). On the other hand, low-calorie ketogenic diet (LCKD) is a KD with a calorie content > 800 kcal and < total energy expenditure (TEE), with 30–50 g of carbohydrates, normoproteic and with a lipid content > 40 g. Lastly, the KD or iso-calorie ketogenic diet (ICKD) includes a calorie content equal to TEE, 30–50 g of carbohydrates and about 70–80% of lipids [21,22,23]. The KD has been used primarily for the treatment of therapy-resistant epilepsy in children [24]. Of note, more restrictive ketogenic approaches, such as the VLCKD, have attracted attention not only for rapid weight loss but also for improved endocrine features, cardiometabolic risk profile, and reproductive function [25,26,27]. From an endocrine and reproductive perspective, its success in patients with endocrine diseases of the female reproductive tract and obesity may be due to some fundamental aspects: rapid and effective weight loss, particularly of fat mass (FM) and visceral adipose tissue, its antioxidant and anti-inflammatory properties and the reduction of insulin resistance [28,29,30,31].
Given the increasing use of VLCKD not only in obesity but also in other related diseases, this literature review will provide the latest scientific evidence of its possible use in the female endocrine-reproductive field as well, and a practical guide to its use in these patients. In this narrative review, we will firstly define and classify obesity, noting which methods are most widely used for body composition analysis. Subsequently, we will explore the beneficial effects of KDs in women life stages, such as the fertile and reproductive period, as well as menopause. Afterwards, we will investigate the potential benefits of the KDs in major disorders of the female reproductive system, such as PCOS, and the potential use of the KDs in the most common cancers of the female reproductive system, such as breast and endometrial cancer.
Definition of Obesity, its Classification and Methodologies to Detect Body Composition
The World Health Organization (WHO) defines obesity as a chronic condition characterized by excess body fat that can cause medical, psychological, physical, social and economic problems and in 1997 declared obesity as a major public health problem and a global epidemic [32]. The prevalence of adult overweight and obesity has increased worldwide since the 1980s, with no decrease in any country during the 33 years of recorded data [33]. Of note, obesity prevalence is increasing among adolescents and children, thus representing a serious public health problem, affecting all demographic groups to a greater or lesser extent. Considering the alarming increase of subjects with overweight and obesity, the WHO has coined the term ‘Globesity’ to describe this phenomenon that has reached the numbers of a global epidemic. Among adults, the prevalence of obesity was generally higher for women than for men in all age brackets. Obesity has become a major contributor to the global burden of chronic diseases affecting virtually all age and socio-economic groups worldwide [34, 35]. Furthermore, it is now recognized that obesity increases the risk of multiple metabolic diseases such as hyperlipidemia, insulin resistance, T2DM, hypertension, atherosclerosis and cardiovascular complications; however, obesity is also correlated with rising infertility and an increase of endocrine disorders including endometriosis and PCOS [36].
Body Composition Assessment
Body mass index (BMI) is the assessment tool used worldwide to estimate the degree of overweight or obesity [37]. According to BMI, individuals are allocated to five different categories as: 18.5–24.9 kg/m2 for normal range, 25.0–29.9 kg/m2 for overweight, 30.0–34.9 kg/m2 for class 1-obesity, 35.0–39.9 kg/m2 for class 2-obesity and equal or greater than 40.0 kg/m2 for class 3-obesity [38]. However, measuring BMI alone has proven inadequate to help clinicians to assess and manage obesity-related health risks in their patients; in fact, BMI does not provide indications about individual’s body composition and adiposity distribution, and today it is well established that abdominal obesity is correlated with increased cardiovascular risk, T2DM, and even some female cancers [37, 39,40,41].
Adipose tissue distribution differs between genders. Men mainly tends to accumulate visceral fat, resulting in the classical android body shape, which is highly correlated with increased cardiovascular risk. On the other hand, women display higher percent body fat and deposit it in a different pattern, with more adipose tissue in the subcutaneous depot, before menopause, a characteristic that offers protection from the negative consequences associated with obesity and metabolic syndrome [42]. After menopause, fat deposition and accumulation shift in favor of visceral deposition. Such modification is accompanied by a parallel increase in metabolic risk reminiscent of that observed in men [42]. Therefore, evaluation of body composition is primarily important to assess the health risk of individuals with obesity and subsequently to target and tailor nutritional treatment.
Several methods can be used to evaluate body composition, with different outcomes on precision and accuracy.
Anthropometric measurements are noninvasive quantitative measurements including height, weight, body circumferences and skinfold thickness [43].
Waist circumference (WC) is a simple method of assessing abdominal obesity that is easily standardized and clinically applicable. According to the National Center for Health Statistics (NCHS), WC should be measured at the tightest point or, when the tightest point is not visible, at an intermediate level between the lower edge of the rib cage and the iliac using a non-stretchable measuring tape [44]. Patients should stand with feet together, on a plane parallel to the ground, with their hands on hips. During the assessment, the patient should breathe normally, and the tape might not compress the skin and should be parallel to the floor [44]. Current guidelines recommend using a single WC threshold for Caucasian women > 88 cm to indicate high WC and increased cardiovascular and T2DM risk [45, 46]. In addition, from the combination of WC and hip circumference, the waist to hip ratio (WHR) can be obtained, the most commonly used index of abdominal obesity. For women, the WHO states that abdominal obesity is defined as a WHR above 0.85 [47]. Thus, WC allows further refinement of the adverse health risk characterized by BMI, and this measurement should be included in stratification of health risk associated with obesity. Resistance to the routine inclusion of WC in clinical practice not only ignores evidence of its utility but also fails to take advantage of the opportunity to counsel patients regarding the higher-risk phenotype of obesity. In addition, measurement of both BMI and WC provides unique opportunities to track the benefits of treatment and the efficacy of interventions for obesity and related metabolic diseases.
Skinfold thicknesses measure subcutaneous body fat at various regions of the body, including triceps, biceps, subscapularis, and suprailiac [48]. Equations are available to calculate total body fat from these measurements (usually for research purposes). The exact technique may vary, but in general the measurement of skinfolds is achieved by grasping the skin 2 cm away from the measurement site [48]. Despite standard measurement techniques, skinfold testing has high variability and has had limited use in clinical settings to date. In addition, skinfold measurement in individuals with obesity is difficult and rarely used [49]. In fact, particularly in subjects with severe obesity, the skinfold may be larger than the caliper can measure; the fold of skin and fat compresses with repeated measurements; and careless use of the caliper causes pain, bruising, and damage to the subjects’ skin [49].
Imaging techniques (dual-energy x-ray absorptiometry, computed tomography, magnetic resonance, and ultrasound) are the most advanced methods for determining body composition [50]. However, they are not feasible in clinical practice because they are expensive, time-consuming, or expose patients to radiation [50].
Bioelectrical impedance (BIA) is widely used in clinical practice and research studies to determine body composition, providing information about FM, fat free mass (FFM), and body water (total, intracellular, and extracellular water), on the basis of equations used to estimate body hydration [51]. In the context of VLCKD protocol, BIA could be used to estimate body composition modifications in terms of FFM maintenance during weight loss. Importantly, monitoring muscle mass could allow early detection of muscle loss and then correction of inadequate protein intake. In addition, phase angle (PhA) has been reported to be an important parameter to monitor during VLCKD. Indeed, PhA is a BIA-derived index that can be used to identify inflammation in various clinical settings [29, 52]. Recently, in a cohort of 260 women (aged 18–69 years, BMI 25.0–50.9 kg/m2), a significant decrease in plasma c-reactive protein concentrations was observed after 31 days of the active phase of VLCKD, whereas the PhA increased. Interestingly, a significant inverse relationship was observed between c-reactive protein and PhA, independent of confounding factors (BMI, WC, age, and physical activity). Therefore, monitoring PhA could be a useful tool to assess changes in the inflammatory status of patients treated with VLCKD and to avoid blood draws and expensive biochemical tests [29].
Ketogenic Diets in Women Life Stages
Ketogenic Diets and Fertility
Infertility is defined as the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse. According to Borght and Wyns, infertility affects between 8 and 12% of couples of reproductive age worldwide [53]. The WHO estimates that 48 million couples and 186 million individuals live with infertility globally [54].
As reported by Skoracka and colleagues, female infertility contributes to 35% of overall infertility cases [55]. The main factors influencing the possibility of spontaneous conception are the age of female partner and infertility linked to endocrine dysfunction [56]. The chance of achieving a spontaneous pregnancy decreases with age before conception. The decline in female fertility begins as early as 25–30 years of age and the median age of last childbirth is 40–41 years in most study populations experiencing natural fertility [56]. Disease-related infertility can affect both sexes and be gender-specific. Factors affecting female fertility include premature ovarian failure, PCOS, endometriosis, uterine fibroids and endometrial polyps [57]. Weight before conception is a major risk factor for fertility outcomes and it is well established that weight loss improves fertility in overweight and obese women [58]. Many overweight women struggling with fertility have co-morbidities. It has been estimated that 75% of infertile women who suffer from overweight or obesity have PCOS, which in itself poses additional challenges to fertility due to disturbances in insulin resistance, sex steroid metabolism and regularity of menstrual cycles [59].
KDs appears to have positive effects as it leads to rapid weight loss, particularly of FM, thus resulting in a reduction in chronic low-grade inflammation induced by dysfunctional adipose organ. Furthermore, reduction in exogenous carbohydrate consumption induces lower insulin production, with improvement in hyperinsulinism and insulin resistance, metabolic alterations commonly observed in infertile women [60].
According to Arbour and colleagues, obesity may negatively affect different stages of woman’s reproductive life, including early menarche, fertility, pregnancy and menopause, as a result of hormonal imbalances and insulin resistance [61]. Moreover, weight loss has positive effects on fertility as well as on the chances of successful assisted reproductive technologies (ART) [62,63,64]. A retrospective cohort study conducted by Moragianni and colleagues showed that women with obesity are 68% less likely to have a live baby after the first cycle of ART than women without obesity [65]. Furthermore, obesity is correlated with the need for higher doses of assisted reproductive therapy drugs and lower rates of efficacy at each stage of the in vitro fertilization (IVF) process [65]. According to McGrice and Porter, weight loss can improve fertility and pregnancy outcomes, particularly with regard to IVF [58]. Benefits for this cohort of patients include more regular menstrual cycles, better quality embryos available for transfer, lower drug dosage and the need for fewer treatment cycles. In a retrospective cohort study, Russell and colleagues investigated if the nutritional components of IVF patient’s daily diet impact blastocyst development and pregnancy outcomes [66]. In this study, 120 patients completed a dietary log and had a fresh or frozen blastocyst transfer. Forty-eight patients were identified with an average daily protein intake > 25% vs. 72 patients who had < 25%. Patients eating > 25% proteins had significant increase in blastocyst development of 54.3% vs. 38.5% (p < 0.001) and pregnancy rates 66.6% (32/48) vs. 31.9% (23/72) (p < 0.005). Patients eating < 40% carbohydrate had a 63.2% (31/49) vs. 33.8% (22/71) pregnancy rate (p < 0.001). Protein intake of > 25% combined with < 40% carbohydrate had an 80% (29/36) pregnancy rate. According to the authors, blastocyst development with improved pregnancy rates may be directly related to nutritional components in a patient’s diet. Patients undergoing IVF with poor blastocyst development may benefit from increasing their average daily protein intake to > 25% and lowering their carbohydrate to < 40% [66].
In a prospective study of 170 women who collectively underwent 233 IVF/ICSI cycle, Chavarro and colleagues showed that overweight and obesity were related to lower live birth rates in women undergoing ART [62]. The authors confirmed that the presence of overweight or obesity at baseline was associated with lower live birth rates [adjusted live birth rate = 23% (14–36%)]. In addition, participants with obesity at baseline were associated with a lower frequency of positive β-human chorionic gonadotropin (hCG). The highest positive β-hCG, pregnancy and live birth rates were observed among women with a BMI between 20.0 and 22.4 kg/m2. Moreover, short-term weight loss was associated with a higher proportion of metaphase II oocytes retrieved. The adjusted proportion of metaphase II eggs was 91% (87–94%) for women who lost 3 kg or more and 86% (81–89%) for women whose weight remained stable (p = 0.002). This association was stronger among women with overweight or obesity at baseline [62].
When fertility represents the main target of infertile patients, particularly at more advanced ages, it is critical to rapidly improve metabolic, nutritional and endocrine conditions in order to facilitate ovulation and pregnancy.
In a systematic review by McGrice and Porter, it was confirmed that reducing carbohydrate load could reduce circulating insulin levels, improve hormonal imbalance and result in ovulation resumption to improve pregnancy rates in infertile women [58]. To this end, the authors’ findings suggest that low-carbohydrate diets may optimize fertility in some clinical groups, particularly in overweight and obese women with PCOS [58].
The application of ketogenic protocols in women with overweight or obesity and infertility is based on the possibility to reduce insulin levels, improving hormonal balance, restoring ovulatory cycles and improving oocyte and embryo quality, as well as pregnancy rates even in subjects undergoing IVF [58, 67, 68]. In particular, as already mentioned, KDs have proven higher efficacy in terms of rapidity of weight loss, dampening of insulin resistance and induction of ovulation, when compared to standard hypocaloric diets also resulting in increased sex hormone binding globulin (SHBG), reduced blood levels of free testosterone, reduced luteinizing hormone to follicle stimulating hormone (LH/FSH) ratio [69, 70].
SHBG is a glycoprotein produced by the liver that binds sex steroids with high affinity and specificity. According to Qu and Donnelly, there is a negative correlation between circulating SHBG levels and markers of insulin resistance [71]. In fact, in vitro studies indicate that SHBG may down-regulate the PI3K/AKT pathway involved in the development of local and systemic insulin resistance [72]. Decreased SHBG levels increase the bioavailability of androgens, which in turn leads to progression of ovarian pathology, anovulation and the phenotypic characteristics of PCOS. Insulin resistance is often accompanied by nutrient oversupply and high dietary intake of monosaccharides can induce low serum SHBG levels through increasing hepatic lipogenesis [73]. It has been shown that dietary fructose increases levels of the enzymes involved in de novo lipogenesis, even more strongly than high fat diet, and that fructose metabolism stimulating de novo lipogenesis is a central abnormality in NAFLD [71]. In a study conducted by Paoli and colleagues, 14 overweight women with diagnosis of PCOS underwent to KD with phytoextracts for 12 weeks [60]. The characteristic reversal of the LH/FSH ratio was observed at the beginning of the study and disappeared after 12 weeks (pre 2.00 ± 0.30 vs post 1.15 ± 0.20; p < 0.001). Compared to basal values, there was also a significant decrease in plasma concentrations of LH (pre 10.24 ± 1.43 vs post 6.41 ± 1.46; p < 0.001), total testosterone (pre 47.43 ± 6.08 ng/dL vs post 40.71 ± 5.77 ng/dL; p < 0.001), free testosterone (pre 0.96 ± 0.60 pg/mL vs post 0.56 ± 0.30 pg/mL; p = 0.009), percentage of free testosterone (pre 2.05 ± 1.33% vs post 2.05 ± 1.33%; p = 0.033) and dehydroepiandrosterone sulfate (pre 2.13 ± 0.26 μg/mL vs post 1.70 ± 0.20 μg/mL; p < 0.001). Estradiol levels were risen (pre 139.80 ± 14.93 pg/mL vs post 191.90 ± 38.80 pg/mL; p < 0.001), so did progesterone (pre 12.16 ± 1.41 ng/dL vs post 21.06 ± 1.86 ng/dL; p < 0.001). SHBG increased significantly from 26.3 ± 7.9 to 34.1 ± 8.7 nmol/L; p < 0.001 [60].
Moreover, according to Cincione and colleagues, KDs determine a reduction in blood glucose levels, insulinemia and an improvement in insulin sensitivity, which consequently led to a reduction in androgen production, whereas the contextual reduction of FM reduced the acyclic production of estrogens deriving from the aromatization in the adipose tissue of the androgen excess, with an improvement of the LH/FSH ratio [69]. This last ratio improves through the reduction of the excess of LH thanks to a relative increase in FSH. Lastly, KDs resulted in an increase in SHBG with a consequent reduction in bioactive free testosterone, thus contributing to a further improvement of hyperandrogenism [69].
Ketogenic Diet and Postmenopause
Menopause is a physiological condition that is diagnosed in the absence of menses for at least 1 year [74]. Indeed, according to Landgrend and colleagues, clinical menopause is diagnosed when a woman has not menstruated for 12 months due to the loss of ovarian follicular activity [75].
During a woman’s fertile life, the average level of total estrogens is 100–250 pg/mL [76]. On the other hand, the concentration of circulating E2 decreases to 10 pg/mL after menopause. Such drastic hormonal reduction is associated with pathological conditions related to menopause, such as sleep disorders, urogenital atrophy, osteopenia and osteoporosis, sexual dysfunction, CVD, cancer, metabolic disorders and obesity [77]. In particular, women display increased risk of developing CVD after menopause due to estrogen deficiency and dysregulation of lipid metabolism [78].
According to Yasui and colleagues, menopausal symptoms, bone loss, changes in lipid profiles and reduction of insulin sensitivity, are frequently observed in women during the menopausal transition, due to an abrupt decrease in circulating estrogen level [79]. On the other hand, circulating levels of testosterone and dehydroepiandrosterone sulfate gradually decrease with age in postmenopausal women, although transient increases have been observed during the menopausal transition [79]. In fact, transient increases of testosterone level have been suggested to be associated with increased risk of CVD, increased triglyceride, and metabolic syndrome [80].
Moreover, menopause induces an increase in body weight, with redistribution of lipids from subcutaneous to visceral depot, resulting in increased inflammatory markers and circulating levels of low-density lipoprotein cholesterol (LDL-C) and reduced insulin sensitivity [81]. Indeed, as reported by Ko and Kim, estrogens, particularly E2, exerts a protective role on the cardiovascular system and is mainly produced in the ovaries through a process involving LDL-C as a substrate [76]. During menopause, circulating LDL-C cannot be used in estrogens synthesis, resulting in decreased estrogens production. Therefore, menopause is associated with increased LDL-C circulating levels and increased CVD [76]. Metabolic alterations associated with weight gain and fat accumulation in menopause increase the risk of metabolic syndrome, as well as cardiovascular and neurodegenerative diseases, induce an increase in peripheral tissues aromatase activity and promote systemic inflammation, synergizing with age-related processes [82, 83]. The application of ketogenic protocols such as very low energy diet (VLED) and VLCKD to postmenopausal women with overweight or obesity is able to promote the reduction of total body weight and visceral adipose tissue with a concomitant preservation of lean mass [84]. In the TEMPO Diet Randomized Clinical Trial, 101 postmenopausal women, aged 45 to 65 years with BMI from 30 to 40 kg/m2, who were at least 5 years after menopause, were recruited. Participants were randomized to either 12 months of moderate (25–35%) energy restriction with a food-based diet (moderate intervention group) or 4 months of severe (65–75%) energy restriction with a total meal replacement diet followed by moderate energy restriction for an additional 8 months (severe intervention group). Compared with the moderate group at 12 months, the severe group lost more weight (effect size, − 6.6 kg; 95% CI, − 8.2 to − 5.1 kg); moreover, whole-body FM (effect size, − 5.5 kg; 95% CI, − 7.1 to − 3.9 kg), abdominal subcutaneous adipose tissue (effect size, − 1890 cm3; 95% CI, − 2560 to − 1219 cm3), and visceral adipose tissue (effect size, − 1389 cm3; 95% CI, − 1748 to − 1030 cm3) loss were also greater for the severe group than for the moderate group at 12 months [84].
Ford and colleagues analyzed data from the Women’s Health Initiative Observational Study, considering four dietary patterns among postmenopausal women aged 49–81 years [mean 63.6 (SD 7.4) years]: a low-fat diet, a reduced-carbohydrate diet, a Mediterranean-style diet and a diet consistent with the US Department of Agriculture’s Dietary Guidelines for Americans (DGA) [85]. At the end of the study, the reduced-carbohydrate diet was inversely related to weight gain (OR 0.71; 95% CI 0.66 to 0.76), whereas the low-fat (OR 1.43; 95% CI 1.33 to 1.54) and DGA (OR 1.24; 95% CI 1.15 to 1.33) diets were associated with increased risk of weight gain. By baseline weight status, the reduced-carbohydrate diet was inversely related to weight gain among women who were normal weight (OR 0.72; 95% CI 0.63 to 0.81), overweight (OR 0.67; 95% CI 0.59 to 0.76) or class I obesity (OR 0.63; 95% CI 0.53 to 0.76) at baseline. The low-fat diet was associated with increased risk of weight gain in women who were normal weight (OR 1.28; 95% CI 1.13 to 1.46), overweight (OR 1.60; 95% CI 1.40 to 1.83), class I obesity (OR 1.73; 95% CI 1.43 to 2.09) or class II obesity (OR 1.44; 95% CI 1.08 to 1.92) at baseline. The authors concluded that a low-carb diet can reduce the risk of weight regain in postmenopausal women compared to a low-fat diet [7].
Accordingly, KDs reduced fasting glycemia, fasting insulin, homeostasis model assessment of insulin resistance: in fact, the severe reduction of carbohydrates, leading to a reduction in plasma insulin levels, induces a reduction in lipogenesis processes in favor of lipolytic ones, resulting in a massive utilization of adipocyte triglycerides for energy purposes [86].
In fact, Boden and colleagues conducted a study enrolling patients with T2DM and obesity, demonstrating that the application of KD for 2 weeks induced the reduction of fasting glycaemia from 7.5 to 6.3 mmol/l and the increase of insulin sensitivity by 75% [87].
Additionally, KDs, particularly VLCKD determines a reduction of insulin levels and a concomitant improvement in plasma lipids by inhibition of HMG-CoA reductase [88, 89]: in fact, the improvement of insulin resistance has positive effects on lipid metabolism through the action on 3-hydroxy-3-methylglutaryl coenzyme A reductase and striking effects on lipoprotein size and subclass particle concentrations [30]. Furthermore, KDs promote the reduction of inflammatory pathways [89,90,91]: according to Paoli and colleagues, that beta-hydroxybutyrate (β-OHB) inhibits NLRP3/inflammasome activation [92]. In fact, β-OHB inhibits NLRP3/inflammasome activation through the reduction of K+ efflux from macrophages and the inhibition of the inflammasome assembly. Moreover, β-OHB-dependent inhibition of IL-1β and IL-18 secretion in human monocytes has been documented. Lastly, KDs could promote the reduction of reactive oxygen species (ROS) in vitro and in vivo [93]. According to Stafford and colleagues, KD reduced ROS production in tumor cells. Gene expression profiling demonstrated that the KD induces an overall reversion to expression patterns seen in non-tumor specimens. Notably, genes involved in modulating ROS levels and oxidative stress were altered, including those encoding cyclooxygenase 2, glutathione peroxidases 3 and 7, and periredoxin 4. To demonstrate the ability of ketones to quantitatively reduce ROS in cultured GL261 cells, the authors treated them with either 2 mM β-OHB/acetoacetate or 10 mM β-OHB/acetoacetate for 24 h prior to ROS analysis using 20 μM 2′, 7′-dichlorofluorescein diacetate (DCF). Tumor cells had high levels of ROS as determined by DCF fluorescence, and the application of either 2 mM or 10 mM ketones resulted in a statistically significant (p < 0.001) decrease in the DCF signal, demonstrating the quantitative reduction of ROS in these cell [93].
Ketogenic Diets in Main Disorders of the Female Reproductive Tract
Ketogenic Diets and PCOS
PCOS is the most common endocrine disease among women in reproductive age, affecting 10 to 15% of women worldwide [94]. The diagnostic criteria include two out of three features: hyperandrogenism, polycystic ovaries on ultrasound and menstrual irregularities [95]. In addition to hormonal changes in gonadotropins and estrogens, obesity, insulin resistance with compensatory hyperinsulinemia and a chronic low-grade inflammatory state often coexist in PCOS [96]. In fact, in women with PCOS, LH concentration increases in relation to FSH, resulting in excessive androgen production. Physiologically, FSH stimulates ovarian follicle maturation and estrogen secretion. In addition, FSH also increases the activity of aromatase, the enzyme responsible for the conversion of androgens into estrogens [96]. Furthermore, insulin acts by increasing the production of androgens (with direct action on the theca cells) and decreasing the hepatic synthesis of the main testosterone-binding protein, resulting in testosterone circulating in the unbound active form. According to Shirazi and colleagues, women with PCOS have abnormal gonadotropin concentration and great androgen biosynthesis from the adrenal and ovaries, stimulated by high levels of insulin, irrespective of body weight [97]. Both metabolic and endocrine alterations of PCOS contribute to the development of metabolic syndrome, T2DM and infertility [98].
Dietary intervention and physical activity also have a positive influence in reducing insulin resistance, body weight and low-grade chronic inflammation. Despite the importance of lifestyle modifications, to date there is still no consensus regarding preferable nutritional treatment for women with PCOS. Since obesity worsens the clinical presentation of PCOS, according to the recommendations of the International Evidence-based Guideline for the Assessment and Management of PCOS, weight management is the main treatment strategy in women with obesity and PCOS. Recent studies have shown that a VLCKD can lead to weight loss and improved insulin sensitivity in PCOS [99, 100]. Paoli and colleagues showed that KD, through improvement of hyperinsulinemia and body composition, contributed to the normalization of the clinical feature in PCOS [60]. Mavropoulos and colleagues conducted a pilot study investigating metabolic and endocrine effects of a low-carbohydrate, KD on overweight and women with obesity and PCOS for 6 months [70]. The authors reported that LCKD showed a significant reduction in body weight (− 12%), free testosterone (− 30%), LH/FSH ratio (− 36%), and basal insulin − 3%) [70].
Hence, improved lifestyle, attention to diet and body weight control are essential building blocks for fertility. According to Barrea and colleagues, high carbohydrate intake and low-grade inflammation influence the development of insulin resistance and hyperandrogenism, thus influencing the pathophysiology of PCOS [1].
According to recent studies, insulin resistance is a condition present in both women with PCOS and with or without obesity [101,102,103].
Another important effect of KDs for PCOS is the activation of AMPK and SIRT-1, even in the absence of caloric deprivation. Once activated, SIRT1 and AMPK produce beneficial effects on glucose homeostasis and improve insulin sensitivity [60].
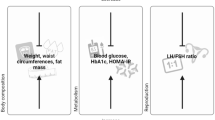
When fertility represents the main target of PCOS patients, particularly at more advanced ages, it is critical to rapidly improve metabolic, nutritional and endocrine conditions in order to facilitate ovulation and pregnancy. KDs have proven higher efficacy in terms of rapidity of weight loss, dampening of insulin resistance and induction of ovulation, when compared to standard hypocaloric diets. In order to effectively tailor dietary treatment, it would be appropriate to apply different types of KDs, depending on body weight, BMI, body composition with particular attention to the percentage of adipose tissue, as reported in Fig. 1. In particular, when obesity or overweight are present, VLCKD represents an extremely effective nutritional tool to obtain a rapid and persistent weight loss and hence improve all clinical conditions in patients with PCOS.
Ketogenic Diets and Tumors
According to recent evidence, cancer represents the second cause of death worldwide, after CVD, in the total population for both sexes combined, and the most commonly diagnosed cancer worldwide is female breast cancer [104, 105]. In 2018, breast cancer represented the first cause of cancer-related death in women worldwide [106]. Interestingly, in 2017, the Center of Disease Control estimated that 40% of all cancers were caused by overweight and obesity (55% in women and 24% in men) [107]. Accordingly, the excess of adipose tissue increases the risk of death in both premenopausal and postmenopausal patients with breast cancer, decreasing overall survival and reducing the effectiveness of cancer treatments [7, 108]. Distinguishing the breast cancer burden by menopausal status is of particular interest, due the opposing role of estrogens in influencing breast cancer development in obesity [109]. In premenopausal women, obesity is associated with a lower risk of estrogens receptor positive (ER+) breast cancer and a higher risk of triple‐negative breast cancers (ER, progesterone receptor, PR, and human epidermal growth factor receptor 2 (HER2). On the other hand, in postmenopausal women, obesity is associated with a markedly higher risk of ER+ breast cancer [110]. The different influence of obesity on ER+ breast cancer development, depending on menopausal status, is explained by hormonal changes, in terms of circulating estrogen, before and after menopause [111, 112]. Ovarian 17β-estradiol (E2) is the main circulating estrogen in the reproductive age. After menopause, E2 circulating levels are markedly reduced and estrone (E1) represents the primary form of estrogen, resulting from the conversion of adrenal androstenedione by aromatase, occurring in peripheral tissues, including adipose tissue and breast. Of note, adipose tissue is the major component of the postmenopausal breast and increased aromatization is observed in obese women [113]. Moreover, it is well known that dysfunctional adipocytes promote low-grade inflammation in obesity and cancer-associated adipocytes recruit and activate macrophages through CCL2/IL-1β/CXCL12 signaling pathway, promoting in turn stromal vascularization and angiogenesis and facilitating tumor progression [114, 115]. It is important to highlight that those patients with breast cancer gain weight even after the diagnosis of cancer, losing muscle mass and developing sarcopenic obesity, displaying negative prognostic effect [116]. Ovarian and endometrial cancer are also associated with obesity, especially in postmenopausal women, due to hormonal changes affecting insulin and IGF-I, all of which are increased in obesity [117]. Although treatment against cancer is mostly based on radio and chemotherapy, there is a great attention to non-pharmacological therapies, including diet therapy, as a tool to modify the cellular metabolism of cancer cells [118]. Growing scientific evidence defines cancer as a metabolic disease [117]. Indeed, the high rate proliferation of cancer cells is mostly related to glucose metabolism than oxygen consumption; the shift from oxidative phosphorylation to anaerobic glycolysis (i.e., the Warburg Effect) is considered as a hallmark of cancer cells [119]. KDs has been applied for over 80 years as an effective adjuvant therapy in refractory epilepsy [120]; however, since 1990s, KDs has been also used to treat metabolic disease (such as obesity and T2DM), CVD cancer [121]. The therapeutic potential of KDs in oncology is related to their ability to impair mitochondrial function, reduce the synthesis of reactive oxygen, decrease chronic inflammation and delay tumor growth, angiogenesis and vascularization of tumor environment [122, 123]. Considering the relevant contribution of inflammation to cancer development, the use of a diet or dietary components (omega 3 fatty acids, ketone bodies, soluble fiber and resistant starch), that reduces inflammation, may reveal as precious tools in cancer prevention and treatment [124]. Finally, KD protocols ensure a valid protein supply in order to satisfy the metabolic needs of the patients, causing weight loss without losing lean body mass. This aspect is critical to maintain performance status in patients with cancer, and to avoid malnutrition, especially in the context of oncological treatments [118].
Ketogenic Diets and Breast Cancer
Breast cancer represents a significant cause of morbidity and mortality in women, as already mentioned [104]. According to the American Cancer Society, breast cancer alone contributes to 30% of all female cancers [125]. Several trials investigated the role of KDs as an adjuvant therapy to enhance sensitivity to chemotherapy and radiotherapy in locally recurrent or metastatic breast cancer [126•, 127]. Studies confirmed the feasibility and the safety of KDs in patients affected by breast cancer with positive effects on body composition, physical performance, and quality of life during the rehabilitation phase [128, 129]. However, most of these studies do not use standardized KDs therapeutic protocols, and the nutritional patterns are very heterogeneous, both in terms of ketogenic ratio and calorie intake [130]. VLCKD, through its drastic calorie restriction and very low-carbohydrate content, reduces glucose availability in cancer cells, impairing the pro-tumorigenic microenvironment and promoting the death of tumor cells through a pro-apoptotic mechanism [123], as represented in Fig. 2.
However, in vivo, the effectiveness of VLCKD in the treatment of breast cancers has been poorly explored. In this scenario, VLCKD may represent a valuable tool for female cancer prevention in women with obesity; moreover, it could be a valuable tool in women with overweight/obesity and a previous history cancer, in order to reduce body weight, visceral adipose tissue, inflammation and the subsequent risk of recurrence. VLCKD represents a time-limited (~ 12 weeks) nutritional pattern that mimics fasting through a marked restriction of daily carbohydrate intake (lower than 30 g/day), with a daily protein intake approximately 1.2–1.5 g/kg of ideal body weight, a fixed amount of fat (20 g/day, mainly from olive oil and omega-3 fatty acids), and a total daily energy intake < 800 kcal [21].
In patients with previous breast cancer, it would also be interesting to assess the VLCKD protein quality favoring vegetable proteins; in fact, according to Pan and colleagues, higher vegetable protein intake was associated with lower breast cancer incidence and lower risk of death after breast cancer, decreasing cancer-related growth factors, such as insulin and IGF-1 [131].
Ketogenic Diets and Endometrial Cancer
Endometrial cancer is the sixth most prevalent cancer in women worldwide [132]. As reported by Braun and colleagues, main risk factors for endometrial cancer are represented by unopposed estrogens therapy, early menarche, late menopause, infertility or failure to ovulate, and PCOS [133]. Additional risk factors are increasing age, obesity, hypertension, T2DM, nulliparity, smoking and hereditary non-polyposis colorectal cancer [133]. According to Onstad and colleagues, the incidence and mortality of endometrial cancer continue to grow, and this trend is a result of the worldwide obesity epidemic [134]. Indeed, more than half of endometrial cancers are currently attributable to obesity, which is recognized as an independent risk factor for this disease [134]. In fact, visceral adipose accumulation promotes proinflammatory milieu, along with deficiency of protective immune cell types in the endometrium, potentially contributing to endometrial cancer risk [135]. Moreover, adipose tissue is responsible for peripheral aromatization of adrenal androgens to estrogens, which, in turn, stimulates endometrium proliferation [8]. During the reproductive stage, such effect is regulated by cyclical progesterone release and regular menstrual bleeding. On the other hand, physiological progesterone decrease, occurring after menopause, together with fat accumulation, contributes to unopposed hyper estrogenic state in obesity, thus increasing the risk of endometrial carcinogenesis [136] (Fig. 2). In addition, evidence of a causal association between obesity-related hyperinsulinemia and endometrial cancer has been provided. Endometrial cancer cells express insulin receptors and both insulin and IGF-1 display mitogenic and antiapoptotic activity, thus potentially contributing to progression of cancer [137, 138]. Accordingly, elevated fasting insulin levels are associated with increased prevalence of endometrioid adenocarcinoma in postmenopausal women. Indeed, elevated insulin circulating levels induce a reduction in SHBG and IGF-binding proteins availability, thus promoting endometrium stimulation by free estrogens and IGF-1 [139].
Given the well-established cancer cells dependence on glucose and insulin availability, KDs is a valid strategy, in association with pharmacological treatment, to counteract cancer proliferation [140]. In fact, glucose and insulin deprivation occurring in patients undergoing a KDs promotes a metabolic environment, which is not suitable for cancer cells growth [141]. In 2018, a randomized controlled trial examining the effects of a 12-week KD in women with ovarian or endometrial cancer, demonstrated for the first time that KD is effective in promoting visceral fat loss, maintaining lean body mass, and decreasing cancer-related growth factors, such as insulin and IGF-1 [117]. Importantly, the same group found that KD eases symptoms due to cancer-related fatigue, along with improved physical function, increased energy, and reduced specific food cravings, thus improving the quality of life in women with ovarian or endometrial cancer [142].
Furthermore, according to Kokts-Porietis and colleagues, obesity at the time of endometrial cancer diagnosis was associated with increased recurrence and all-cause mortality among endometrial cancer survivors [143]. In this context, VLCKD may represent a preventive measure in postmenopausal women with obesity, to reduce visceral adipose tissue, inflammatory status, growth factors such as insulin and IGF-1, and to reduce the risk of endometrial cancer. Moreover, it could be a valuable tool in women with prior endometrial cancer and obesity to reduce body weight, visceral adipose tissue, circulating insulin levels and risk of recurrence.
When is it Possible to Set Up a VLCKD Protocol?
According to Consensus Statement of Italian Society of Endocrinology (SIE), short- and medium-term studies support the use of VLCKD in individuals with obesity (BMI ≥ 30.0 kg/m2) or overweight (BMI 25.0–29.9 kg/m2) with abdominal obesity (WC > 88 cm in women) [21]. In addition, VLCKD may be recommended when excess body weight is related to comorbidities including T2DM, NAFLD, obstructive sleep apnea syndrome, and PCOS [20, 21].
Conversely, some conditions represent absolute contraindications to the use of VLCKD. In particular, pregnancy and lactation, or childhood and adolescence are physiological conditions during which VLCKD should be avoided. Pathophysiological conditions include the presence of comorbidities such as hepatic, renal, cardiac and respiratory insufficiency, type 1 diabetes, myocardial infarction or recent cerebrovascular stroke, severe psychiatric disorders. In addition, eating disorders and alcohol and substance abuse are contraindications for VLCKD [20, 21].
VLCKD Protocol: Step by Step
As stated in the SIE position statement, the VLCKD protocol is divided into different stages, involving the drastic reduction of carbohydrate content for the first 8–12 weeks, to induce nutritional ketosis [21]. The initial stage of VLCKD is characterized by a very low-calorie diet (650–800 kcal/day), low in carbohydrate (< 30 g daily from vegetables), and fat (only 20 g per day, derived also from olive oil). The amount of high-biological-value protein ranged between 1.2 and 1.4 g per each kg of ideal body weight in order to preserve lean mass: protein can be derived from conventional foods (such as eggs, meat or fish) or meal replacements can be used [21, 23]. Scientific evidence suggests that meal replacement in the first active ketogenic phase should be recommended to ensure a safe, effective, and controlled administration of VLCKD [30]. In fact, with the use of single-portioned meal replacement meals, the calibration of the diet is more accurate, and the content of calories, macronutrients and micronutrients needed by the patient can be set more precisely and individually [30].
Therefore, it would seem more appropriate to set up a VLCKD protocol with the use of meal replacements to determine greater safety, efficacy, and compliance in patients with obesity, with preference given to freeze-dried meal replacements, which generally have a higher protein content and lower fat and carbohydrate content. This would ensure a higher degree of weight loss and a better adherence [22].
Being a very low caloric nutritional pattern, it is recommended to supplement patients with micronutrients (vitamins, such as complex B vitamins, vitamin C and E, minerals, including potassium, sodium, magnesium, calcium; and omega-3 fatty acids) according to international recommendations [22]. Hydration is very important at this early stage: about 2–2.5 L of water per day are needed. In addition, it is very important to use low glycemic index vegetables to reach the necessary fiber quota [22]. After this first active ketogenic phase, low-calorie diet is prescribed (LCD stage): at this point, different food groups will be progressively reintroduced. In particular, carbohydrates are gradually reintroduced, starting from foods with the lowest glycemic index (such as dairy products and fruits). The daily calorie intake of LCD diet ranges between 1000 and 1200 kcal/day, with carbohydrates amount of 60–100 g. Then, a hypocaloric balanced diet is sustained, with a caloric intake between 1300 and 1400 kcal and a carbohydrate intake of 130–150 g; legumes are reintroduced [22].
Lastly, a maintained hypocaloric balanced diet is sustained, following a Mediterranean diet with a caloric intake between 1500 and 1800 kcal, with the reintroduction of low glycemic index cereal. This last step, through the acquisition of correct eating habits, is crucial for maintaining long-term results [22]. As reported, it is essential for the patient with obesity to lose at least 15% of body weight and maintain this loss over the long term to reduce cardiometabolic risks [22, 23]. Energy intake is provided by 30% fat, 45% carbohydrate, and 25% protein, as for the traditional Mediterranean diet. More in detail, protein intake should be 1–1.2 g/kg of desirable weight (desirable weight: weight corresponding to a BMI of 22.5 kg/m2). Recommended carbohydrate sources should be high-fiber foods with slow-absorption starches, avoiding high simple sugars-intake (max 10%) and favoring the intake of vegetables, fruit, cereals, and legumes. This nutritional profile allows patients to continue their nutritional re-education while maintaining the achieved weight loss [22, 23].
Finally, we emphasize that both the KD and the Mediterranean diet have specific advantages for endocrine disorders of the female reproductive system. As we have previously seen, KD can promote weight loss, improve insulin sensitivity, and potentially improve hormonal balance, but it can cause nutrient deficiencies, limit food choices, and lack long-term research [144]. The Mediterranean diet offers a nutrient-rich approach, is beneficial for heart health and has anti-inflammatory properties, but may have limited effects on weight loss, requires individual variation and has risks related to processed foods [145]. However, the two nutritional approaches can be combined to enhance beneficial effects for patients with endocrine disorders of the reproductive system. Very recently, Verde and colleagues demonstrated in 318 women with overweight/obesity that a high adherence to the Mediterranean diet prior to the start of VLCKD improves the efficacy of the latter in terms of both weight loss and improvements in body composition [146•]. The authors attributed these results to the presence of bioactive compounds in the Mediterranean diet, which together and in the context of Mediterranean diet could lead to a favorable metabolic set-up for the onset of more effective ketosis [146•]. Furthermore, it is important to remind that a Mediterranean-style nutritional approach represents the last step (maintenance phase) of the VLCKD protocol [23]. For this reason, both nutritional approaches could be appropriately combined in women with endocrine disorders of the reproductive system to maximize the beneficial effects of the dietary intervention as part of their management.
Conclusions
As inflammatory status, oxidative stress and excess adipose tissue could have a negative effect on fertility and are associated with the development of female diseases including PCOS and cancer, finding successful therapeutic strategies is of utmost importance. Furthermore, given the preeminent role of obesity as significant risk factor for the development of endocrine disease in women, any therapeutical strategy should include specific dietary guidelines aimed at reducing inflammatory parameters. The use of KDs has shown significantly favorable effects in reducing inflammation and achieving weight loss, particularly FM and visceral adipose tissue. Ketogenic protocol is effective in nutritional and cardiometabolic rehabilitation in various pathophysiological conditions in women, particularly menopause, cancer and insulin resistance. KDs should be prescribed under close medical supervision. Furthermore, the diet should be tailored to the individual patient, customizing the calorie and macronutrient content. Since there are few published studies on the effect of KDs on PCOS, fertility, and breast and endometrial cancers (Table 1), well-designed controlled studies are needed to establish the best KD protocol in terms of diet period, macro- and micronutrient combinations, use of supplements, and carbohydrate reintroduction.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Barrea L, Marzullo P, Muscogiuri G, Di Somma C, Scacchi M, Orio F, et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018;31(2):291–301.
Chetrite GS, Feve B. Preface to special issue on Peripheral and central control of human reproduction: endocrine aspects–part 2. Horm Mol Biol Clin Investig. 2016;25(1):1–3.
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis Nat Rev Dis Primers. 2018;4(1):9.
Marinelli S, Napoletano G, Straccamore M, Basile G. Female obesity and infertility: outcomes and regulatory guidance. Acta Biomed. 2022;93(4): e2022278.
Karczewski J, Sledzinska E, Baturo A, Jonczyk I, Maleszko A, Samborski P, et al. Obesity and inflammation. Eur Cytokine Netw. 2018;29(3):83–94.
Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):277–89.
Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41.
McDonald ME, Bender DP. Endometrial cancer: obesity, genetics, and targeted agents. Obstet Gynecol Clin North Am. 2019;46(1):89–105.
Laudisio D, Muscogiuri G, Barrea L, Savastano S, Colao A. Obesity and breast cancer in premenopausal women: current evidence and future perspectives. Eur J Obstet Gynecol Reprod Biol. 2018;230:217–21.
Laudisio D, Castellucci B, Barrea L, Pugliese G, Savastano S, Colao A, et al. Mediterranean diet and breast cancer risk: a narrative review. Minerva Endocrinol (Torino). 2021;46(4):441–52.
Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, et al. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–29.
Pugliese GD, Barrea LD, Laudisio DD, Aprano SD, Castellucci BD, Framondi LD, et al. Mediterranean diet as tool to manage obesity in menopause: a narrative review. Nutrition. 2020;79–80: 110991.
Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr. 2021;61(7):1201–11.
Barrea L, Vetrani C, Altieri B, Verde L, Savastano S, Colao A, et al. The importance of being a ‘lark’ in post-menopausal women with obesity: a ploy to prevent type 2 diabetes mellitus? Nutrients. 2021;13(11).
Vetrani C, Barrea L, Rispoli R, Verde L, De Alteriis G, Docimo A, et al. Mediterranean diet: what are the consequences for menopause? Front Endocrinol (Lausanne). 2022;13: 886824.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–66.
Barrea L, Pugliese G, Muscogiuri G, Laudisio D, Colao A, Savastano S. New-generation anti-obesity drugs: naltrexone/bupropion and liraglutide. An update for endocrinologists and nutritionists. Minerva Endocrinol. 2020;45(2):127–37.
Ryan DH. Guidelines for obesity management. Endocrinol Metab Clin North Am. 2016;45(3):501–10.
Velapati SR, Shah M, Kuchkuntla AR, Abu-Dayyeh B, Grothe K, Hurt RT, et al. Weight regain after bariatric surgery: prevalence, etiology, and treatment. Curr Nutr Rep. 2018;7(4):329–34.
Trimboli P, Castellana M, Bellido D, Casanueva FF. Confusion in the nomenclature of ketogenic diets blurs evidence. Rev Endocr Metab Disord. 2020;21(1):1–3.
Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Invest. 2019;42(11):1365–86.
Muscogiuri G, Barrea L, Laudisio D, Pugliese G, Salzano C, Savastano S, et al. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. J Transl Med. 2019;17(1):356.
Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L, et al. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–45.
Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):3–5.
Castellana M, Biacchi E, Procino F, Casanueva FF, Trimboli P. Very-low-calorie ketogenic diet for the management of obesity, overweight and related disorders. Minerva Endocrinol (Torino). 2021;46(2):161–7.
Mongioi LM, Cimino L, Condorelli RA, Magagnini MC, Barbagallo F, Cannarella R, et al. Effectiveness of a very low calorie ketogenic diet on testicular function in overweight/obese men. Nutrients. 2020;12(10).
Tragni E, Vigna L, Ruscica M, Macchi C, Casula M, Santelia A, et al. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: a study in a real-world setting. Nutrients. 2021;13(6).
Barrea L, Caprio M, Watanabe M, Cammarata G, Feraco A, Muscogiuri G, et al. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit Rev Food Sci Nutr. 2022:1–17.
Barrea L, Muscogiuri G, Aprano S, Vetrani C, de Alteriis G, Varcamonti L, et al. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int J Obes (Lond). 2022;46(9):1591–7.
Basciani S, Camajani E, Contini S, Persichetti A, Risi R, Bertoldi L, et al. Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab. 2020;105(9).
Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, Bussade I, et al. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front Endocrinol (Lausanne). 2020;11:607.
James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond). 2008;32(Suppl 7):S120–6.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Haslam D. Obesity in primary care: prevention, management and the paradox. BMC Med. 2014;12:149.
Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400–9.
Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50(3):117–28.
Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17.
Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160(14):2117–28.
Freuer D, Linseisen J, O'Mara TA, Leitzmann M, Baurecht H, Baumeister SE, et al. Body fat distribution and risk of breast, endometrial, and ovarian cancer: a two-sample Mendelian randomization study. Cancers (Basel). 2021;13(20).
Goh LG, Dhaliwal SS, Welborn TA, Lee AH, Della PR. Anthropometric measurements of general and central obesity and the prediction of cardiovascular disease risk in women: a cross-sectional study. BMJ Open. 2014;4(2): e004138.
Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Curr Diab Rep. 2018;18(9):69.
Chomtho S. 1.2.1 Clinical evaluation and anthropometry. World Rev Nutr Diet. 2022;124:7–15.
Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO expert consultation on waist circumference and waist-hip ratio. Eur J Clin Nutr. 2010;64(1):2–5.
Madden AM, Smith S. Body composition and morphological assessment of nutritional status in adults: a review of anthropometric variables. J Hum Nutr Diet. 2016;29(1):7–25.
Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–89.
WHO. Waist circumference and waist-hip ratio: report of a WHO expert consultation [cited 2023 Apr 25]. Available from: https://www.who.int/publications/i/item/9789241501491.
Stewart A, Eston R. Skinfold thickness measurement. Br J Nutr. 1997;78(6):1040–1.
Gray DS, Bray GA, Bauer M, Kaplan K, Gemayel N, Wood R, et al. Skinfold thickness measurements in obese subjects. Am J Clin Nutr. 1990;51(4):571–7.
Riccardi G, Aggett P, Brighenti F, Delzenne N, Frayn K, Nieuwenhuizen A, et al. PASSCLAIM--body weight regulation, insulin sensitivity and diabetes risk. Eur J Nutr. 2004;43 Suppl 2:II7-II46.
Smith S, Madden AM. Body composition and functional assessment of nutritional status in adults: a narrative review of imaging, impedance, strength and functional techniques. J Hum Nutr Diet. 2016;29(6):714–32.
Barrea L, Muscogiuri G, Laudisio D, Di Somma C, Salzano C, Pugliese G, et al. Phase angle: a possible biomarker to quantify inflammation in subjects with obesity and 25(OH)D deficiency. Nutrients. 2019;11(8).
Sibbald B. The future supply of registered nurses in Canada. Can Nurse. 1998;94(1):22–3.
WHO. Infertility [cited 2023 Apr 25] Available from https://www.who.int/news-room/fact-sheets/detail/infertility.
Skoracka K, Ratajczak AE, Rychter AM, Dobrowolska A, Krela-Kazmierczak I. Female fertility and the nutritional approach: the most essential aspects. Adv Nutr. 2021;12(6):2372–86.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Recent advances in medically assisted conception. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1992;820:1–111.
McGrice M, Porter J. The effect of low carbohydrate diets on fertility hormones and outcomes in overweight and obese women: a systematic review. Nutrients. 2017;9(3).
Nikokavoura EA, Johnston KL, Broom J, Wrieden WL, Rolland C. Weight loss for women with and without polycystic ovary syndrome following a very low-calorie diet in a community-based setting with trained facilitators for 12 weeks. Diabetes Metab Syndr Obes. 2015;8:495–503.
Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104.
Arbour MW, Stec M, Walker KC, Wika JC. Clinical implications for women of a low-carbohydrate or ketogenic diet with intermittent fasting. Nurs Womens Health. 2021;25(2):139–51.
Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98(1):109–16.
Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes. 2014;4(2):61–8.
Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review Obes Rev. 2014;15(10):839–50.
Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102–8.
Russell JB, Abboud C, Williams A, Gibbs M, Pritchard S, Chalfant D. Does changing a patient's dietary consumption of proteins and carbohydrates impact blastocyst development and clinical pregnancy rates from one cycle to the next? Fertility and Sterility. 2012;98(3).
Kulak D, Polotsky AJ. Should the ketogenic diet be considered for enhancing fertility? Maturitas. 2013;74(1):10–3.
Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril. 2006;86(1):227–9.
Cincione RI, Losavio F, Ciolli F, Valenzano A, Cibelli G, Messina G, et al. Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome. Int J Environ Res Public Health. 2021;18(23).
Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005;2:35.
Qu X, Donnelly R. Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. Int J Mol Sci. 2020;21(21).
Feng C, Jin Z, Chi X, Zhang B, Wang X, Sun L, et al. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol Endocrinol. 2018;34(7):567–73.
Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117(12):3979–87.
Verde L, Barrea L, Vetrani C, Frias-Toral E, Chapela SP, Jayawardena R, et al. Chronotype and sleep quality in obesity: how do they change after menopause? Curr Obes Rep. 2022;11(4):254–62.
Landgren BM, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM. Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab. 2004;89(6):2763–9.
Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 2020;12(1).
Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, et al. Prevention of diseases after menopause. Climacteric. 2014;17(5):540–56.
Sobenin IA, Myasoedova VA, Orekhov AN. Phytoestrogen-rich dietary supplements in anti-atherosclerotic therapy in postmenopausal women. Curr Pharm Des. 2016;22(2):152–63.
Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M. Androgen in postmenopausal women. J Med Invest. 2012;59(1–2):12–27.
Torrens JI, Sutton-Tyrrell K, Zhao X, Matthews K, Brockwell S, Sowers M, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of women’s health across the nation. Menopause. 2009;16(2):257–64.
Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14.
Mumusoglu S, Yildiz BO. Metabolic syndrome during menopause. Curr Vasc Pharmacol. 2019;17(6):595–603.
Pu D, Tan R, Yu Q, Wu J. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric. 2017;20(6):583–91.
Seimon RV, Wild-Taylor AL, Keating SE, McClintock S, Harper C, Gibson AA, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2(10): e1913733.
Ford C, Chang S, Vitolins MZ, Fenton JI, Howard BV, Rhee JJ, et al. Evaluation of diet pattern and weight gain in postmenopausal women enrolled in the Women’s Health Initiative Observational Study. Br J Nutr. 2017;117(8):1189–97.
Basolo A, Magno S, Santini F, Ceccarini G. Ketogenic diet and weight loss: is there an effect on energy expenditure? Nutrients. 2022;14(9).
Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403–11.
Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88(4):1617–23.
Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315.
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256–64.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9.
Paoli A, Gorini S, Caprio M. The dark side of the spoon - glucose, ketones and COVID-19: a possible role for ketogenic diet? J Transl Med. 2020;18(1):441.
Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond). 2010;7:74.
Barrea L, Frias-Toral E, Verde L, Ceriani F, Cucalon G, Garcia-Velasquez E, et al. PCOS and nutritional approaches: differences between lean and obese phenotype. Metabol Open. 2021;12: 100123.
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38 e25.
Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73(5):473–87.
Shirazi FKH, Khodamoradi Z, Jeddi M. Insulin resistance and high molecular weight adiponectin in obese and non-obese patients with Polycystic Ovarian Syndrome (PCOS). BMC Endocr Disord. 2021;21(1):45.
Orio F, Muscogiuri G, Nese C, Palomba S, Savastano S, Tafuri D, et al. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: an uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:214–9.
Frias-Toral E, Garcia-Velasquez E, de Los Angeles Carignano M, Rodriguez-Veintimilla D, Alvarado-Aguilera I, Bautista-Litardo N. Polycystic ovary syndrome and obesity: clinical aspects and nutritional management. Minerva Endocrinol (Torino). 2022;47(2):215–41.
Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117–25.
Daghestani MH. Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int J Gynaecol Obstet. 2018;142(2):162–9.
Pande AR, Guleria AK, Singh SD, Shukla M, Dabadghao P. beta cell function and insulin resistance in lean cases with polycystic ovary syndrome. Gynecol Endocrinol. 2017;33(11):877–81.
Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(2):277–85.
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021.
Lane J, Brown NI, Williams S, Plaisance EP, Fontaine KR. Ketogenic diet for cancer: critical assessment and research recommendations. Nutrients. 2021;13(10).
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8.
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–97.
Qureshi R, Picon-Ruiz M, Aurrekoetxea-Rodriguez I, Nunes de Paiva V, D'Amico M, Yoon H, et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 2020;31(6):1154–72 e9.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5).
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–36.
Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124(3):698–712.
Nimrod A, Ryan KJ. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab. 1975;40(3):367–72.
Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73(19):6080–93.
Vitiello L, Ferraro E, De Simone S, Gatta L, Feraco A, Racioppi L, et al. CXCL12 prolongs naive CD4+ T lymphocytes survival via activation of PKA, CREB and Bcl2 and BclXl up-regulation. Int J Cardiol. 2016;224:206–12.
Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(8 Suppl):2277–87.
Cohen CW, Fontaine KR, Arend RC, Alvarez RD, Leath CA III, Huh WK, et al. A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr. 2018;148(8):1253–60.
Barrea L, Caprio M, Tuccinardi D, Moriconi E, Di Renzo L, Muscogiuri G, et al. Could ketogenic diet “starve” cancer? Emerging evidence. Crit Rev Food Sci Nutr. 2022;62(7):1800–21.
Schwartz L, Supuran CT, Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anticancer Agents Med Chem. 2017;17(2):164–70.
Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(5–6):431–9.
Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab. 2020;33:102–21.
Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645.
Seyfried TN, Mukherjee P, Iyikesici MS, Slocum A, Kalamian M, Spinosa JP, et al. Consideration of ketogenic metabolic therapy as a complementary or alternative approach for managing breast cancer. Front Nutr. 2020;7:21.
Elisia I, Krystal G. The pros and cons of low carbohydrate and ketogenic diets in the prevention and treatment of cancer. Front Nutr. 2021;8: 634845.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
• Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr. 2021;40(3):751–8. This RCT evaluated the effects of KD in patients with advanced breast cancer undergoing chemotherapy. KD reduced TNF-α, insulin and tumour size, but further research is needed on the effects in patients with metastases.
Wang Y, Jing MX, Jiang L, Jia YF, Ying E, Cao H, et al. Does a ketogenic diet as an adjuvant therapy for drug treatment enhance chemotherapy sensitivity and reduce target lesions in patients with locally recurrent or metastatic Her-2-negative breast cancer? Study protocol for a randomized controlled trial. Trials. 2020;21(1):487.
Kammerer U, Klement RJ, Joos FT, Sutterlin M, Reuss-Borst M. Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients. 2021;13(3).
Klement RJ, Weigel MM, Sweeney RA. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. 2021;40(6):4267–74.
Klement RJ, Champ CE, Kammerer U, Koebrunner PS, Krage K, Schafer G, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III-final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020;22(1):94.
Pan K, Larson JC, Prentice RL, Mortimer JE, Neuhouser ML, Manson JE, et al. Protein intake by source and breast cancer incidence and mortality: the women's health initiative. JNCI Cancer Spectr. 2020;4(6):pkaa101.
Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28.
Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93(6):468–74.
Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34(35):4225–30.
Naqvi A, MacKintosh ML, Derbyshire AE, Tsakiroglou AM, Walker TDJ, McVey RJ, et al. The impact of obesity and bariatric surgery on the immune microenvironment of the endometrium. Int J Obes (Lond). 2022;46(3):605–12.
Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43.
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):921–9.
Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92(18):1472–89.
Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, et al. Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. J Natl Cancer Inst. 2015;107(9).
Intlekofer AM, Finley LWS. Metabolic signatures of cancer cells and stem cells. Nat Metab. 2019;1(2):177–88.
Klement RJ, Kammerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011;8:75.
Cohen CW, Fontaine KR, Arend RC, Soleymani T, Gower BA. Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: a randomized, controlled trial. Nutrients. 2018;10(9).
Kokts-Porietis RL, Elmrayed S, Brenner DR, Friedenreich CM. Obesity and mortality among endometrial cancer survivors: a systematic review and meta-analysis. Obes Rev. 2021;22(12): e13337.
O’Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. 2020;292:119–26.
Mentella MC, Scaldaferri F, Ricci C, Gasbarrini A, Miggiano GAD. Cancer and Mediterranean diet: a review. Nutrients. 2019;11(9).
• Verde L, Dalamaga M, Capo X, Annunziata G, Hassapidou M, Docimo A, et al. The antioxidant potential of the Mediterranean diet as a predictor of weight loss after a very low-calorie ketogenic diet (VLCKD) in women with overweight and obesity. Antioxidants (Basel). 2022;12(1). In this observational study, adherence to the Mediterranean diet was positively correlated with the effectiveness of VLCKD in treating obesity by improving weight loss and body composition.
Acknowledgements
Figure generation has been performed using BioRender.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This work was supported by a funding from the Italian Ministry of Health (Ricerca Corrente).
Author information
Authors and Affiliations
Contributions
Conceptualization: Elisabetta Camajani, Alessandra Feraco, Luigi Barrea and Giovanna Muscogiuri; Methodology: Elisabetta Camajani, Alessandra Feraco, Luigi Barrea and Giovanna Muscogiuri; Writing—original draft preparation: Elisabetta Camajani, Alessandra Feraco, Ludovica Verde, Marco Marchetti, Eleonora Moriconi; Writing—review and editing: Luigi Barrea, Giovanna Muscogiuri, Massimiliano Caprio and Francesca Marino; Supervision: Annamaria Colao. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elisabetta Camajani, Alessandra Feraco, and Ludovica Verde are co-first names.
Massimiliano Caprio, Giovanna Muscogiuri, and Luigi Barrea are co-last names.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Camajani, E., Feraco, A., Verde, L. et al. Ketogenic Diet as a Possible Non-pharmacological Therapy in Main Endocrine Diseases of the Female Reproductive System: A Practical Guide for Nutritionists. Curr Obes Rep 12, 231–249 (2023). https://doi.org/10.1007/s13679-023-00516-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00516-1