Summary
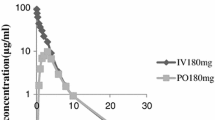
Cefetamet pivoxil (pivoxyl) belongs to the class of orally absorbed prodrug esters that are hydrolysed to the active compound (Cefetamet) on first pass through the gut wall, the liver or both. Intravenously administered cefetamet is eliminated predominantly unchanged in the urine by glomerular filtration. Systemic and renal clearance values for cefetamet were 8.16 and 7.14 L/h (136 and 119 ml/min), respectively. Plasma protein binding is 22% and steady-state volume of distribution (0.29 L/kg) corresponds roughly to the extracellular water space. Cefetamet pivoxil exhibits a significant positive food effect (40 vs 50%) after oral administration. Hence, it is recommended that cefetamet pivoxil is taken with meals. The food effect is not related to changes in gastric pH, since a mixture of aluminium and magnesium hydroxide (Maalox®) or ranitidine did not affect the bioavailability. The predominant renal elimination of cefetamet and the lack of effect of age and disease on cefetamet pivoxil absorption make dosage adjustment in relation to renal dysfunction simple and reliable. The recommended dosage regimen for susceptible bacterial strains in adults with normal renal function is cefetamet pivoxil 500mg twice daily. The same dosage schedule can be used in children, with dose adjustments made according to body surface area.
Similar content being viewed by others
References
Angehrn P, Hohl P, Then RL. In vitro antibacterial properties of cefetamet and in vivo activity of its orally absorbable ester derivative, cefetamet pivoxil. European Journal of Clinical Microbiology and Infectious Diseases 8: 536–543, 1989
Balant L, Dayer P, Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clinical Pharmacokinetics 10: 101–143, 1985
Bequé P, Garabedian N, Quinet B, Baron S. Penetration of cefixime in the tonsils of pediatrics. Pathologie Biologie 37: 433–436, 1989
Blouin RA, Kneer J, Ambros RJ, Stoeckel K. Influence of antacid and ranitidine on the pharmacokinetics of oral cefetamet pivoxil. Antimicrobial Agents and Chemotherapy 34: 1744–1748, 1990
Blouin RA, Kneer J, Stoeckel K. Pharmacokinetics of intravenous cefetamet (Ro 15-8074) and oral cefetamet pivoxil (Ro 15-8075) in young and elderly subjects. Antimicrobial Agents and Chemotherapy 33: 291–296, 1989
Brown RD, Campoli-Richards DM. Antimicrobial therapy in neonates, infants and children. Clinical Pharmacokinetics 17 (Suppl. 1): 105–115, 1989
Cullmann W, Dick W. Cefpodoxime: comparable evaluation with other orally available cephalosporins. With a note on the role of β-lactamases. Zentralblatt für Bakteriologie 273: 501–517, 1990
Deppermann KM, Garbe C, Hasse K, Koeppe P, Lode H. Comparative pharmacokinetics of cefotiam hexetil, cefuroxime axetil, cefixime, cephalexin and the effect of H2-blockers, standard breakfast and antacids on the bioavailability of cefotiam hexetil. Abstract 24. Paul Ehrlich Society for Chemotherapy, Bonn, 1989
Drusano GL. Role of pharmacokinetics in outcome of infections. Antimicrobial Agents and Chemotherapy 32: 289–297, 1988
Faulkner RD, Yacobi A, Barone JS, Kaplan SA, Silber BM. Pharmacokinetic profile of cefixime in man. Pediatric Infectious Disease Journal 6: 963–970, 1987
Finn A, Stranghn A, Meyer M, Chubb J. Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharmaceutics and Drug Disposition 8: 519–526, 1987
Gerber AU, Craig WA, Brugger HP, Feller C, Vastola AP, et al. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. Journal of Infectious Diseases 147: 910–917, 1983
Grellet J, Couraud L, Sauy MC. Roche G. Diffusion of cefixime in the human lung. Presse Médicale 18: 1589–1592, 1989
Hayton WL, Kneer J, Blouin RA, Stoeckel K. Pharmacokinetics of intravenous cefetamet and oral cefetamet pivoxil in patients with hepatic cirrhosis. Antimicrobial Agents and Chemotherapy 34: 1318–1322, 1990
Hayton WL, Walstad RA, Thurmann-Nielsen E, Kufaas T, Kneer J, et al. Pharmacokinetics of intravenous cefetamet and oral cefetamet pivoxil in children. Antimicrobial Agents and Chemotherapy 35: 720–725, 1991
Hughes GS, Heald DL, Barker KB, Patel RK, Spillers CR, et al. The effect of gastric pH and food on the pharmacokinetics of a new oral cephalosporin, cefpodoxime proxetil. Clinical Pharmacology and Therapeutics 46: 674–685, 1989
Kneer J, Tam YK, Blouin RA, Frey FJ, Keller E, et al. Pharmacokinetics of intravenous cefetamet and oral cefetamet pivoxil in patients with renal insufficiency. Antimicrobial Agents and Chemotherapy 33: 1952–1957, 1989
Koup JR, Dubach UC, Brandt R, Wyss R, Stoeckel K. Pharmacokinetics of cefetamet (Ro 15-8074) and cefetamet pivoxil (Ro 15-8075) after intravenous and oral doses in humans. Antimicrobial Agents and Chemotherapy 32: 573–579, 1988
Nichols RL, Wikler MA, McDevitt JT, Lentnek AL, Hosutt JA. Coagulopathy associated with extended-spectrum cephalosporins in patients with serious infections. Antimicrobial Agents and Chemotherapy 31: 281–285, 1987
Perea EJ, Ayarra J, Garcia Iglesias MC, Garcia Luque J, Loscertales J. Penetration of cefuroxime and ceftazidime into human lungs. Chemotherapy 34: 1–7, 1988
Sattler FR, Weitekamp MR, Ballard JO. Potential for bleeding with the new beta-lactam antibiotics. Annals of Internal Medicine 105: 924–931, 1986
Schentag JJ. Clinical significance of antibiotic tissue penetration. Clinical Pharmacokinetics 16 (Suppl. 1): 25–31, 1989
Schentag JJ, Smith IL, Swanson DJ, De Angelis C, Fracasso JE, et al. Role for dual individualization with cefmenoxime. American Journal of Medicine 77 (Suppl. 6A): 43–50, 1984
Sommers DeK, Van Wyk M, Moncrieff J, Schoeman HS. Influence of food and reduced gastric acidity on the bioavailability of bacampicilline and cefuroxime axetil. British Journal of Clinical Pharmacology 18: 535–539, 1984
Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Ziegler WH. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clinical Pharmacology and Therapeutics 29: 650–657, 1981
Tarn YK, Kneer J, Dubach UC, Stoeckel K. Pharmacokinetics of cefetamet pivoxil (Ro 15-8075) with ascending oral doses in normal healthy volunteers. Antimicrobial Agents and Chemotherapy 33: 957–959, 1989
Tarn YK, Kneer J, Dubach UC, Stoeckel K. Effect of timing of food and fluid volume on cefetamet pivoxil absorption in healthy normal volunteers. Antimicrobial Agents and Chemotherapy 34: 1556–1559, 1990
Tozer TN. Concepts basic to pharmacokinetics. Pharmacology and Therapeutics 12: 109–131, 1981
Tremblay D, Antrobus H, Lenfant B, Coussediere D, Dupront A. Pharmacokinetics of cefpodoxime in young and elderly volunteers after single oral doses of cefpodoxime proxetil. Abstr. 619, International Congress of Infectious Diseases, Montreal, 1990
Uschida E, Oguchi K, Hisaoka M, Kobayashi S, Kai K, et al. Effects of ranitidine, metodopromide, and anisotropine meth-ylbromide on the availability of Cefpodoxime proxetil (CS-807) in Japanese healthy subjects. Japanese Journal of Clinical Pharmacology and Therapeutics 19: 573–579, 1988
Williams TF. Aging or disease? Clinical Pharmacology and Therapeutics 42: 663–665, 1987
Wyss R, Bucheli F. Determination of cefetamet and its orally active ester, cefetamet pivoxil, in biological fluids by high-performance liquid chromatography. Journal of Chromatography — Biomedical Applications 430: 81–92, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stoeckel, K. Pharmacokinetics of Intravenous Cefetamet and Oral Cefetamet Pivoxil in Human Subjects. Drug Invest. 3, 291–298 (1991). https://doi.org/10.1007/BF03259741
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03259741