Summary
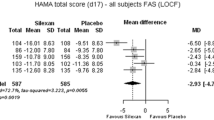
In order to assess the efficacy and tolerability of mirtazapine (Org 3770), 40 outpatients with a primary diagnosis of anxiety states [International Classification of Diseases (ICD-9)] were treated in a randomised, double-blind, placebo-controlled, 4-week study (mirtazapine, n = 20; placebo, n = 20). Daily dosages of mirtazapine were 15 to 25mg. The dosage could be titrated upwards or downwards by 5 mg/day, depending on the clinical response or the development of adverse events, respectively. Anxiety symptoms were assessed at baseline and after 7,14,21 and 28 days of treatment using the Hamilton Anxiety Scale (HAMAS) and the Zung Anxiety Scale (ZAS); global functioning was assessed by the Global Assessment Scale (GAS). Biochemical, haematological and urinary variables were also measured. After 28 days of treatment, mirtazap-ine-treated patients experienced significantly greater improvement from baseline in overall anxiety symptoms and psychic anxiety (assessed by HAMAS and ZAS scores) and in global functioning (assessed by GAS scores) than placebo-treated patients. There were no clinically significant changes in biochemical, haematological or urinary variables with either treatment. Mirtazapine was well tolerated; only one patient withdrew from the study because of adverse events. Sedation was experienced significantly more often by mirtazapine-treated patients, while placebo-treated patients complained significantly more often of insomnia and crying, and had more sleep disturbances. In this study, mirtazapine appeared to be an effective and well tolerated treatment for patients with anxiety symptoms as a primary complaint.
Similar content being viewed by others
References
Clement HH, Gemsa D, Weseman W. The effect of noradrenergic drugs on serotonin metabolism in the nucleus raphe dorsalis of the rat, studied by in vivo voltametry. Eur J Pharmacol 1992; 217: 43–8
de Boer T, Nefkens F, van Helvoirt A. The α2-antagonist Org 3770 enhances serotonin transmission in vivo. Eur J Pharmacol 1994; 253: R5–R6
Nickolson V, Radhakishun F, Sitsen JMA, et al. Remeron (Org 3770), an ‘atypical’ antidepressant with ‘typical’ efficacy [abstract no. 109]. Presented at the 9th World Congress of Psychiatry; Jun 6–12: Brazil: 1993
Bremner JD. A double-blind comparison of Org 3770, amitrip-tyline and placebo in major depression. Submitted
Claghorn JL. A double-blind, placebo-controlled study of Org 3770 in depressed patients. J Affect Dis. In press
Marttila M, Jääskeläinen J, Järvi R, et al. A double-blind study comparing efficacy and tolerability of Org 3770 and doxepin in patients with major depression. Submitted
Richou H, Ruimy P, Charbaut J, et al. A multicentre, double-blind, clomipramine-controlled efficacy and safety study of Org 3770. Submitted
Smith WT, Glaudin V, Panagides J. Mirtazapine vs amitriptyline vs placebo in the treatment of major depressive disorder. Psy-chopharmacol Bull 1990; 26: 191–6
Van Moffaert M, de Wilde D, Vereecken A, et al. A double-blind comparison of Org 3770 vs trazodone in hospitalized patients. Int Clin Psychopharmacol. In press
Zivkov M, de Jongh GD. Org 3770 vs amitriptyline: a 6-week randomised, double-blind multicentre trial in hospitalized depressed patients. Human Psychopharm. In press
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed (DSM-III). Washington DC: American Psychiatric Association, 1980
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd ed. revised (DSM-III-R). Washington DC: American Psychiatric Association, 1987
World Health Organization. The ICD-10 classification of mental and behavioural disorders, clinical descriptions and diagnostic guideline. Geneva: World Health Organization, 1992
Liebowitz MR. Treating the patient with depression and associated anxiety. In: Serotonin: reshaping the treatment of depression. Toronto: The MEDICINE Publishing Foundation Symposium Series (No. 32), 1992: 31–41
Kasper S, Fuger J, Müller HJ. Comparative efficacy of antide-pressants. Drugs 1992; 43 Suppl. 2: 11–23
Paykel ES. Recent developments in antidepressants. Curr Ther Res 1993; 54: 811–9
World Health Organization. Mental disorders: glossary and guide to their classification in accordance with the ninth revision of the international classification of diseases. Geneva: World Health Organization, 1978
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–5
Zung WWK. A rating instrument for anxiety disorders. Psycho-somatics 1971; 12: 371–9
Endicott J, Spitzer RL, Fleiss JL, et al. The Global Assessment Scale: a procedure of measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33: 766–71
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sitsen, J.M.A., Moors, J. Mirtazapine, a Novel Antidepressant, in the Treatment of Anxiety Symptoms. Drug Invest 8, 339–344 (1994). https://doi.org/10.1007/BF03257448
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257448