Abstract
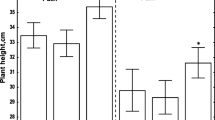
Studies were performed to determine the effect that plant hormones involved with plant senescence (i.e., ethylene and abscisic acid) and photoperiod have on disease development and symptom expression of bacterial ring rot (BRR) caused byCorynebacterium sepedonicum (Spieck & Kotth.) Skapt & Burkh. Potato plants were grown either under a long (14 hr.) or short (10 hr.) photoperiod, while eggplants were grown only under a short (10 hr.) photoperiod. Disease severity ratings of BRR were found to be significantly higher (P=0.05) on potato plants grown under a short photoperiod compared to a long photoperiod. Plant heights of BRR infected plants were found to be significantly greater under the long photoperiod. Endogenous levels of ethylene were found to be significantly (P=0.05) greater in inoculated potato plants grown under a long photoperiod than inoculated plants grown under a short photoperiod. Results suggest that the plant hormones ethylene and abscisic acid do not significantly affect the disease development and symptom expression of BRR.
Compendio
Se llevaron a cabo estudios para determinar el efecto que tienen las hormonas vegetales involucradas en la senescencia de las plantas (v.gr. el etileno y el ácido abscísico) y en el fotoperiodo sobre el desarrollo y expresión de sÍntomas de la pudrición anular bacteriana (BRR) causada porCorynebacterium sepedonicum (Spieck & Kotth.) Skapt and Burkh. Las plantas de papa crecieron bajo un fotoperiodo largo (14 hr) o corto (10 hr), mientras que las de berenjena crecieron solamente bajo un fotoperiodo corto (10 hr). Se encontró que los grados de severidad de la pudrición fueron significativamente mayores (P = 0,05) sobre las plantas de papa crecidas bajo un fotoperiodo corto en comparación con un fotoperiodo largo. Las alturas de las plantas infectadas con BRR fueron significativamente mayores bajo el fotoperiodo largo. Se encontró que los niveles endógenos de etileno fueron significativamente (P=0,05) mayores en las plantas de papa inoculadas y crecidas bajo un fotoperiodo largo que en las que crecieron bajo un fotoperiodo corto. Los resultados sugieren que las hormonas vegetales etileno y ácido abscÍsico no afectan significativamente el desarrollo y la expresión de sÍntomas de la pudrición anular bacteriana (BRR).
Similar content being viewed by others
Literature Cited
Achilea, O., E. Chalutz, Y. Fuchs and I. Rot. 1985. Ethylene biosynthesis and related physiolgocial changes inPénicillium digitatum-infected grapefruit (Citrus paradisi). Phys Plant Pathology 26:125–134.
Ben-Yehoshua, S. and B. Aloni. 1974. Effect of water stress on ethylene production by detached leaves of valencia orange (Citrus sinensis Osbeck). Plant Physiology 53:863–865.
Beyer, E.M., Jr. 1976. A potent inhibitor of ethylene action in plants. Plant Physiology 58:268–271.
Bishop, A.L. and S.A. Slack. 1987. Effect of cultivar, inoculum dose and strain ofClavibacter michiganese subsp.sepedonicum on symptom development in potatoes. Phytopathology 77:1085–1089.
Bishop, A.L. and S.A. Slack. 1987. Effect of inoculum dose and preparation, strain variation, and plant growth conditions on the eggplant assay for bacterial ring rot. Am Potato J 64:227–234.
Coleman, L.W. and C.F. Hodges. 1987. Ethylene biosynthesis inPoa pratensis leaves in response to injury or infection byBipolaris sorokiniana. Phytopathology 77:1280–1283.
Dimond, A.E. and P.E. Waggoner. 1953. The cause of epinastic symptoms in fusarium wilt of tomatoes. Phytopathology 43:663–669.
Freebairn, H.T. and I.W. Buddenhagen. 1964. Ethylene production byPseudomonas solanacearum. Nature, Lond. 202:313–314.
Garner, W.W. and H.A. Allard. 1923. Further studies in photoperiodism, the response of the plant to relative length of day and night. J of Agri Res 23:871–920.
Goodman, R.N., Z. Kiraly and K.R. Wood. 1986. The Biochemistry and Physiology of Plant Disease, p. 245–286. University of Missouri Press, Columbia.
Goto, M., Y. Ishida, Y. Takikawa and H. Hydoo. 1985. Ethylene production by the Kudzu strains ofPseudomonas syringae pv.phaseolicola causing halo blight inPueramia lobata (wild) Ohw: Plant Cell Physiology 26:141–150.
12. Kefeli, V.I. and Ch. Sh. Kayrov. 1971. Natural growth inhibitors, their chemical and physiological properties. Ann Rev of Plant Physiology 22:185–196.
Logsden, C.E. 1967. Effect of soil temperature on potato ring rot. Am Potato J 44:281–286.
Lund, B.M. and L.W Mapson. 1970. Stimulation byErwinia carotovora of the synthesis of ethylene in cauliflower tissue. Biochem Journal 119:251–263.
Mapson, L.W and A.C. Hulme. 1970. The biosynthesis, physiological effects and mode of action of ethylene. In ‘Progess in Phytochemistry’ (Eds. L. Reinhold and Y. Liwschitz) Vol. 2:343–384. Interscience Publishers, London.
McMichael, B.L., W.R. Jordan and R.D. Powell. 1972. An effect of water stress on ethylene production by intact cotton petioles. Plant Physiology 49:658–660.
Melis, R.J.M. and J. van Staden. 1984. Tuberization and hormones. Z. Pflanzenphysiol Bd 113 S pp. 271–283.
Nelson, G.A. 1980. Long term survivial ofC. sepedonicum on contaminated surfaces and in infected potato stems. Am Potato J 57:595–600.
Nelson, G.A. and G.C. Kozub. 1983. Effect of total light energy on symptoms and growth of ring rot-infected red pontiac potato plants. Am Potato J 60:461–468.
Nelson, G.A., W.E. Torfason and F.R. Harper. 1971. Comparison of inoculation methods on ring rot development in potatoes. Am Potato J 48:225–229.
21. Pratt, H.K. and J.D. Goeschl. 1969. Physiological roles of ethylene in plants. Ann Rev of Plant Physiology 20:541–584.
Reid, M.S., J.L. Paul, M.B. Farhoomand, A.K. Kofranek and G. L. Staby. 1980. Pulse treatments with the silver thiosulphate complex extend the vase-life of cut carnations. J Am Soc Hortic Sci, 105:25–27.
Sequeira, L. 1973. Hormone metabolism in diseased plants. Ann Rev of Plant Physiology Vol. 24:353–380.
Schaad, N.W. 1988. Initial identification of common genera, page 3,In: Laboratory guidefor identification of plant pathogenic bacteria, 2nd edition. N.W. Schaad, editor. APS Press 164 pp.
Slack, S.A. and A.L. Bishop. 1984. Bacterial ring rot of potato: A perspective of disease diagnosis and control in North America. Proc No Amer Seed Po Seminar 2:13–26.
Stall, R.E. and C.B. Hall. 1984. Chlorosis and ethylene production in pepper leaves infected byXanthomonas campestris pv.vesicatoria. Phytopathology 74:373–375.
Steadman, J.R. and L. Sequeira. 1970. Abscisic acid in tobacco plants. Tentative identification and its relation to stunting induced byPseudomonas solanacearum. Plant Physiology 45:691–697.
Veen, H. 1983. Silver thiosulphate: an experimental tool in plant science. Scientia Horticulturae 20:211–224.
Wareing, P.F. and I.D.J. Phillips. 1981. Growth and differentiation in plants. 3rd Edition. Peragamon Fress. Pgs. 143–148.
Wareing, P.F. and A.M.V. Jennings. 1979. The hormonal control of tuberization in potato.In: F. Skoog (Ed.) Plant Growth Substances pp. 293–300 Proc 10th Int Conf Springer Verlag, Berlin.
Wiese, M.V. and J.E. DeVay. 1970. Growth regulator changes in cotton associated with defoliation caused byVerticillium albo-atrum. Plant Physiology 45:304–309.
Author information
Authors and Affiliations
Additional information
Published with the approval of the Director of the North Dakota Agricultural Experiment Station as Journal No. 1800.
Rights and permissions
About this article
Cite this article
Kurowski, C.J., Gudmestad, N.C. The effect of ethylene and abscisic acid on symptom expression of bacterial ring rot in eggplant and potato. American Potato Journal 67, 443–459 (1990). https://doi.org/10.1007/BF03044512
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03044512