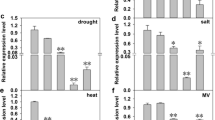
Abstract
We previously demonstrated that both trehalose and LEA protein protect plants from damage by drought, salt, and heat. Here, we compared their effectiveness in preserving photosynthetic capacity under those abiotic stresses. Upon dehydration, the Pmax (maximal photosynthetic rate) of O2 evolution decreased similarly in both nontransformants andotsA plants. Contrastingly, Pmax was maintained at a considerably higher level inCaLEA6 plants. However, no significant differences in Chl fluorescence parameters were observed between transformants and nontransformants. Under salinity stress,CaLEA6 plants were also better thanotsA plants in terms of their values for Pmax, photochemical efficiency of PSII(Fv/Fm), and photochemical quenching (qP). After heat bothotsA andCaLEA6 plants maintained a higher Pmax as well as more favorable Chl fluorescence parameters, although the latter transformant performed slightly better overall. Therefore, despite the comparable effectiveness of trehalose and LEA protein in enhancing tolerance against those abiotic stresses, they confer differential protection in maintaining photosynthetic capacity. Compared with trehalose, the CaLEA6 protein appears to be a more universal and effective agent under those stresses.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- Fm:
-
maximal fluorescence after dark-adaptation
- Fo:
-
initial fluorescence
- Fv:
-
variable fluorescence
- Fv/Fm:
-
maximum photochemical efficiency of PSII
- LEA:
-
late embryo abundant
- NPQ:
-
nonphotochemical quenching of chlorophyll fluorescence
- PEG:
-
polyethylene glycol
- Pmax:
-
maximal photosynthetic rate of O2 evolution
- PS:
-
photosystem
- qP:
-
photochemical quenching of chlorophyll
- T6P:
-
trehalose-6-phosphate
- TPP:
-
trehalose-6-phosphate phosphatase
- TPS:
-
trehalose-6-phosphate synthase
Literature cited
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems 1 and II inSynechococcus sp. Plant Physiol 123: 1047–1056
Angelopoulos K, Dichio B, Xiloyannis C (1996) Inhibition of photosynthesis in olive tree (Olea europaea L.) during water stress and rewatering. J Exp Bot 47: 1093–1100
Argüelles JC (2000) Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch Microbiol 17: 217–224
Baker J, Steel C, Dure III L (1988) Sequence and characterization of 6Lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291
Boyer JS, Bowen BL (1970) Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol 45: 612–615
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2: 48–54
Colaco C, Kampinga J, Roser B (1995) Amorphous stability and trehalose. Science 268: 788
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97: 13430–13435
Define S, Alvino A, Concetta M, Loreto F (1999) Restrictions to carbon dioxide conductance and photosynthesis in spinach leaves recovering from salt stress. Plant Physiol 119: 1101–1106
Dure III L (1993) A repeating 11-mer amino acid motif and plant desiccation. Plant J 3: 363–369
Dure III L, Croch M, Harada J, Ho T-HD, Mundy J, Quatrano RS, Thomas T, Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12: 475–486
Galau GA, Wang HY-C, Hughes DW (1993) Cotton Lea5 and Lea14 encode atypical late embryogenesis-abundant proteins. Plant Physiol 101: 695–696
Garg AK, Kim J-K, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903
Greenway H, Munns R (1980) Mechanisms of salt tolerance in non-halophytes. Annu Rev Plant Physiol 31: 149–190
Holmström KO, Mantyla E, Welin B, Palva ET (1996) Drought tolerance in tobacco. Nature 279: 683–684
Imai R, Chang L, Otha A, Bray EA, Takagi M (1996) A lea-class gene of tomato confers salt and freezing tolerance when expressed inSaccharomyces cerevisiae. Gene 170: 243–248
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 377–403
Jang I-C, Oh S-J, Seo J-S, Choi W-B, Song SI, Kim CH, Kim YS, Seo H-S, Choi YD, Nahm BH, Kim J-K (2003) Expression of a bifunctional fusion of theEscherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131: 516–524
Jun S-S, Choi HJ, YangJY, Hong Y-N (2001) Photosynthetic response to dehydration and high temperature in trehalose-producing transgenic tobacco. 12th International Congress on Photosynthesis. CSIRO Publishing, Queensland, Australia
Jun S-S, Yang JY, Choi HJ, Kim N-R, Park MC, Hong Y-N (2005) Altered physiology in trehalose-producing transgenic tobacco plants: Enhanced tolerance to drought and salinity stresses. J Plant Biol 48: 456–466
Kassen I, Falkenberg R Styrvold OB, Strøm AR (1992) Molecular cloning and physical mapping of theotsBA genes, which encode the osmoregulatory trehalose pathway ofEscherichia coli: Evidence that transcription is activated byKatF (AppR). J Bacteriol 174: 889–898
Kim H-S, Lee JH, Kim JJ, Kim C-H, Jun S-S, Hong Y-N (2005) Molecular and functional characterization ofCaLEA6, the gene for a hydrophobic LEA protein fromCapsicum annuum. Gene 344: 115–123
Lee HY, Jun S-S, Hong Y-N (1998) Photosynthetic responses to dehydration in green pepper (Capsicum annuum L.) leaves. J Photosci 5: 169–174
Lee HY, Jun S-S, Hong Y-N (2004) Increased photoinhibition in dehydrated leaves of hot pepper (Capsicum annuum L.) is not accompanied by an incremental loss of functional PSII. J Plant Biol 47: 83–91
Maitra N, Cushman JC (1994) Isolation and characterization of drought-induced soybean cDNA encoding a D95 family late embryogenesis-abundant protein. Plant Physiol 106: 805–806
Michel BE (1970) Carbowax 6000 compared with mannitol as a suppressant of cucumber hypocotyl elongation. Plant Physiol 45: 507–509
Mohanty P, Boyer JS (1976) Chloroplast response to low water potentials. IV. Quantum yield is reduced. Plant Physiol 57: 704–709
Müller J, Wiemken A, Aeschbacher R (1999) Trehalose metabolism in sugar sensing and plant development. Plant Sci 147: 37–47
Ögren E, Öquist G (1985) Effects of drought on photosynthesis, chlorophyll fluorescence and photoinhibition susceptibility in intact willow leaves. Planta 166: 380–388
Park SH, Jun S-S, An G, Hong Y-N, Park MC (2003) A comparative study on the protective role of trehalose and LEA proteins against abiotic stresses in transgenic Chinese cabbage(Brassica campestris) overexpressingCaLEA orotsA. J Plant Biol 46: 277–286
Pastenes C, Horton P (1996) Effect of high temperature on photosynthesis in beans. I. Oxygen evolution and chlorophyll fluorescence. Plant Physiol 112: 1245–1260
Pellny TK, Ghannoum O, ConroyJP, Schluepmann H, Smeekens S, Andralojc J, Krause KP, Goddijn O, Paul MJ (2004) Genetic modification of photosynthesis withE. coli genes for trehalose synthesis. Plant Biotech J 2: 71–82
Piatkowski D, Schneider K, Salamini F, Bartels D (1990) Characterizationof five abscisic acid-responsive cDNA clones isolated from desiccation-tolerant plantCraterostigma plantagineum and their relationship to other water-stress genes. Plant Physiol 94: 1682–1688
Pilon-Smits EAH, Terry N, Sears T, Kim H, Zayes A, Hwang SB, van Dun K, Voogd E, Verwoerd TC, Krutwagen RWHH, Goddijn OJM (1998) Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J Plant Physiol 152: 525–532
Ramanjulu S, Bartels D (2002) Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ 25: 141–151
Renger G, Schreiber U (1986) Practical applications of fluometric methods to algae and higher plant research,In Govindjee, J Amesz, DC Fork, eds, Light Emission by Plants and Bacteria. Academic Press, Orlando/London, pp 587–619
Ried JL, Walker-Simmons MK (1993) Group 3 late embryogenesis abundant proteins in desiccation-tolerant seedlings of wheat(Triticum aestivum L.). Plant Physiol 102: 125–131
Romero C, Bellés JM, Vayá JL, Serrano R, Culiáñez-Macià FA (1997) Expression of the yeasttrehalose-6-phosphate synthase gene in transgenic tobacco plants: Pleiotropic phenotypes include drought tolerance. Planta 201: 293–297
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis,In E-D Schulze, MM Caldwell, eds, Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, pp 49–70
Soulages JL, Kim KM, Walters C, Cushman JC (2002) Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol 128: 822–832
Swire-Clark GA, Marcotte WR (1999) The wheat LEA protein Em functions as an osmoprotective molecule inSaccharomyces cerevisiae. Plant Mol Biol 39: 117–128
van der Eycken W, de Almeida Engler J, Inzé D, van Montagu M, Gheysen G (1996) A molecular study of root-knot nematode-induced feeding sites. Plant J 9: 45–54
Xu D, Duan X, Wang B, Hong B, Ho DT-H, Wu R (1996) Expression of a late embryogenesis abundant protein gene,HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110: 249–257
Yamane Y, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth Res 52: 57–64
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: Evolution of osmolyte system. Science 217: 1214–1222
Zegzouti H, Jones B, Marty C, Lelièvre J-M, Latché A, Pech J-C, Bouzayen M (1997) ER5, a tomato cDNA encoding an ethylene-responsive LEA-like protein: Characterization and expression in response to drought, ABA and wounding. Plant Mol Biol 35: 847–854
Zhang L, Ohta A, Takagi M, Imai R (2000) Expression of plant group 2 and group 3 lea genes inSaccharomyces cerevisiae revealed functional divergence among LEA proteins. J Biochem 127: 611–616
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jun, S.S., Choi, H.J., Lee, H.Y. et al. Differential protection of photosynthetic capacity in trehalose-and lea protein-producing transgenic plants under abiotic stresses. J. Plant Biol. 51, 327–336 (2008). https://doi.org/10.1007/BF03036134
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03036134