Abstract
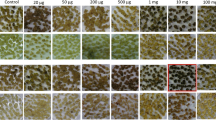
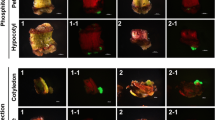
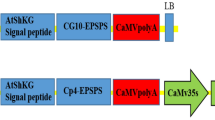
A binary vector devoid of a plant selection-marker gene (designated as pSSA-F) was constructed to overcome bio-safety concerns about genetically modified plants. This vector carried chloroplast-targeted superoxide dismutase (SOD) and ascorbate peroxidase (APX) genes under the control of an oxidative stress-inducible(SWPA2) promoter, and was utilized to transform potato (Solanum tuberosum L.). Integration of these foreign genes into transgenic plants was primarily performed via PCR with genomic DNA. Twelve marker-free transgenic lines were obtained by inoculating stem explants. The maximum transformation efficiency was 6.25% and averaged 2.2%. Successful integration of the SOD and APX genes rendered transgenic plants tolerant to methyl viologen-mediated oxidative stress at the leaf-disc and whole-plant levels. Our findings suggest that this technique for developing selection marker-free transgenic plants is feasible and can be employed with other crop species.
Similar content being viewed by others
Literature Cited
Ahmad R, Kim MD, Back KH, Kim HS, Lee HS, Kwon SY, Murata N, Chung WI, Kwak SS (2008) Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep 27: 687–698
Allen RD, Web RP, Schake SA (1997) Use of transgenic plants to study antioxidant defenses. Free Radic Biol Med 23: 473–479
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasakai R, Kubo A, Tanaka K (2004) Over-expression of ascorbate peroxide in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121: 231–238
Bary EA, Serres JB, Weretilynk (2000) Responses to abiotic stresses,In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, USA, pp 1158–1203
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Christou P, Twyman RM (2004) The potential of genetically enhanced plants to address food insecurity. Nutr Res Rev 17: 23–42
de Vetten N, Wolters A, Raemarkers K, Meer I, Stege R, Heeres E, Heeres P, Visser R (2003) A transformation method for obtaining marker free plants of cross-pollinating and vegetatively propagated crop. Nat Biotechnol 21: 439–442
Ebinuma H, Sugita K, Matsunaga E, Yamado M, Komamine A (2001) System for the removal of selection marker and their combination with a positive marker. Plant Cell Rep 20: 383–392
Gleave AP, Mitra DS, Mudge SR, Morris BAM (1999) Selectable marker-free transgenic plants without sexual crossing: Transient expression of cre recombinase and use of a conditional lethal gene. Plant Mol Biol 40: 223–235
Gong M, Li Y, Chen S (1998) Abscisic acid-induced thermotolerance in maize seedling is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153: 488–496
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinosaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17: 287–291
Khedher MB, Ewing EE (1985) Growth analyses of eleven potato cultivars grown in the green house under long photoperiod with and without heat stress. Amer Potato J 62: 537–554
Kim KY, Kwon SY, Lee HS, Hur Y, Bang JW, Kwak SS (2003) A novel oxidative stress-inducible peroxidase promoter from sweet potato: Molecular cloning and characterization in transgenic tobacco plants and cultured cells. Plant Mol Biol 51: 831 -838
Kim MS, Kim HS, Kim HN, Kim YS, Baek KH, Park YI, Joung H, Jeon JH (2007) Growth and tuberization of transgenic potato plants expressing sense and antisense sequences of Cu/Zn superoxide dismutase from lily chloroplasts. J Plant Biol 50: 490–495
Kim SH, Hamada T (2005) Rapid and reliable method of extracting DNA and RNA from sweetpotato,Ipomoea batatas (L) Lam. Biotech Lett 27: 1841–1845
Koning A (2003) A framework for designing transgenic crops — Science safety and citizen’s concerns. Nat Biotechnol 21: 1274–1279
Li ZX, Moon BP, Burgoyne SA, Guida AD, Liang H, Lee C, Caster CS, Barton JE, Klein TM, Falco SC (2007) A Cre/loxp-mediated self activating gene excision system to produce marker gene free transgenic soybean plants. Plant Mol Biol 65: 329–341
McCord JM, Fridovich I (1969) Superoxide dismutase: An enzymatic function for erythrocuperin (hemocuprin). J Biol Chem 244: 6049–6055
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: Applications, alternatives and biosafety. Biotechnol J 107: 193–232
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880
Newell C, Rozma R, Hinchee MA, Lawson EC, Haley L, Sander R Kaniewski W, Tumer NE, Horsh RB, Fraley RT (1991)Agrobacterium-mediated transformation ofSolanum tuberosum L. cv. Russet Burbank. Plant Cell Rep 10: 30–34
Park JM, Lee YK, Kang BK, Chung WI (2004) Co-transformation using a negative selectable marker gene for the production of selectable marker gene-free transgenic plants. Theor Appl Genet 109: 1562–1567
Ramessar K, Peremarti A, Galera SG, Naqvi SM. Moralejo R Munoz, T. Capell (2007) Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: A case of science not supporting the politics. Transgen Res 16: 261–280
Sen Gupta A, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993) Increased resistance in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90: 1629–1633
Storozhenko S, De Pauw R van Montagu M, Inze D, Kushnir S (1998) The heat-shock element is a functional component of theArabidopsis APX1 gene promoter. Plant Physiol 118:1005–1014
Tang L, Kim MD, Yang KS, Kwon SY, Kim SH, Kim JS, Yun DJ, Kwak SS, Lee HS (2008) Enhanced tolerance of transgenic potato plants overexpressing nucleoside diphosphate kinase 2 against multiple environmental stresses. Transgen Res 17: 705–715
van Camp W, Inze D, van Montagu M (1997) The regulation and function of tobacco superoxide dismutases. Free Radic Biol Med 23: 515–520
Xiang C, Han R Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40: 711–717
Zuo E, Nui QW, Moller SG, Chua NH (2001) Chemical-regulated, site-specific DNA excision in transgenic plants. Nat Biotechnol 19: 157–161
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, R., Kim, YH., Kim, M.D. et al. Development of selection marker-free transgenic potato plants with enhanced tolerance to oxidative stress. J. Plant Biol. 51, 401–407 (2008). https://doi.org/10.1007/BF03036060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03036060