Abstract
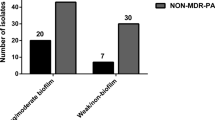
Resistance to 17 antimicrobials, surface hydrophobicity, motility, biofilm, production ofN-acylhomoserine lactone signal molecules (N-butyrylhomoserine lactone andN-3-oxolauroylhomoserine lactone) and response to oxidative stress were analyzed in 47 clinicalPseudomonas aeruginosa strains. In addition to natural resistance, the strains demonstrated the greatest level of resistance to cefotaxime (91.5 %). Isolates in the range of 44.7–57.4 % were resistant to aminoglycosides and ciprofloxacin, of 25.5–36.2 % to cephalosporins. On the other hand, 97.9 % remained susceptible to meropenem, 93.6 % to piperacillin + tazobactam and 87.2% to piperacillin. The majority of the strains (72.3 %) manifested their hydrophilic character. Higher zones of motility showed 12 isolates (in average 54.8 mm) as compared to the others (30.2 mm). Approximately 1/3 of the strains (29.8 %) produced a higher amount of biofilm quantified by measuring the absorbance of solubilized crystal violet (0.20–0.46) than the rest of isolates (0–0.19). All but two strains producedN-3-oxolauroylhomoserine lactone and in 48.9 % of samplesN-butyrylhomoserine lactone were detected. Only four isolates with higher biofilm production showed both types of homoserine lactone. Majority of the strains (70.2 %) manifested higher resistance to H2O2 than the rest of the strains. The group of strains resistant to aminoglycosides and ciprofloxacin revealed a significantly higher number of hydrophobic strains (compared with the sensitive ones). In contrast, higher number of strains sensitive to aminoglycosides and ciprofloxacin or only to ciprofloxacin producedN-butyrylhomoserine lactone and biofilm (compared to the resistant ones). Such association was not found among the rest of the tested parameters. The results indicate that the resistance to antimicrobials inP. aeruginosa isolates was not generally associated with changes in the production of the pathogenicity factors.
Similar content being viewed by others
Abbreviations
- HSL:
-
homoserine lactone(s)
- 3-oxo-C12-HSL:
-
N-3-oxolauroylhomoserine lactone
- C4-HSL:
-
N-butyrylhomoserine lactone
References
Allison D.G., Mc Bain A.J., Gilbert P.: Microbial biofilms: problems of control, pp. 309–327 in D.G. Allison, P. Gilbert, H. Lappin-Scott, M. Wilson (Eds):Community, Structure and Cooperation in Biofilms. Cambridge University Press, Cambridge 2000.
Bonafonte M.A., Solano K., Sesma B., Alvarez M., Montuenga L., Garcia-Ros D., Gamazo C.: The relationship between glycogen synthesis, biofilm formation and virulence inSalmonella enteritidis.FEMS Microbiol.Lett. 191, 31–36 (2000).
Bonfiglio G., Marchetti F.:In vitro activity of ceftazidime, cefepime and imipenem on 1005Pseudomonas aeruginosa clinical isolates either susceptible or resistant to β-lactams.Chemotherapy 46, 229–234 (2000).
Braga P.C., Dalsasso M.: Sub-MIC concentrations of cefodizime interfere with various factors affecting bacterial virulence.J.Antimicrob.Chemother. 45, 15–25 (2000).
Camprubí S., Merino S., Benedí J., Williams P., Tomás M.: Physicochemical surface properties ofKlebsiella pneumoniae.Curr.Microbiol. 24, 31–33 (1992).
Cavallo J.D., Leblanc F., Fabre R., Gerp B.:Pseudomonas aeruginosa: susceptibility to antibiotics and distribution of β-lactam resistance mechanisms.Pathol.Biol. 48, 472–477 (2000).
Černohorská L., Votava M.: Determination of minimal regrowth concentration (MRC) in clinical isolates of various biofilm-forming bacteria.Folia Microbiol. 49, 75–78 (2004).
Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.A., Costerton J.W., Greenberg E.P.: The involvement of cell-to-cell signals in the development of a bacterial biofilm.Science 280, 295–298 (1998).
Edgeworth J.D., Treacher D.F., Eykyn S.J.: A 25-year study of nosocomial bacteremia in adult intensive care unit.Crit.Care Med. 27, 1421–1428 (1999).
Fluit A.C., Verhoef J., Schmitz F.J.:The European Sentry Participants: Antimicrobial resistance in European isolates ofPseudomonas aeruginosa.Eur.J.Clin.Microbiol.Infect.Dis. 19, 370–374 (2000).
Fuqua C., Winans S., Greenberg E.P.: Census and consensus in bacterial ecosystems: the Lux R-Lux I family of quorum sensing transcriptional regulators.Ann.Rev.Microbiol. 50, 727–751 (1996).
Gagniére H., Di Martino P.: α5β1 integrins and fibronectin are involved in adherence of thePseudomonas aeruginosa ER97314 clinical strain to A549 cells.Folia Microbiol. 49, 757–762 (2004).
Gál Z., Szabó D., Kovács P., Hernádi F., Tóth-Martinez B., Rozgonyi F.: β-Lactam susceptibility patterns and investigation of cephalosporin hydrolysing β-lactamases of Gram-negative extraintestinal clinical isolates.Internat.J.Antimicrob.Agents 16, 395–400 (2000).
Gattringer R., Niks M., Ostertag R., Schwarz K., Medvedovic H., Graninger W., Georgopoulos A.: Evaluation ofMidi-Tech automated colorimetric MlC reading for antimicrobial susceptibility testing.J.Antimicrob.Chemother. 49, 651–659 (2002).
Harjai K., Khandwaha R.K., Mittal R., Yadav V., Gupta V., Sharma S.: Effect of pH on production of virulence factors by biofilm cells ofPseudomonas aeruginosa.Folia Microbiol. 50, 99–102 (2005).
Hassett D.J., Schweizer H.P., Ohman D.E.:Pseudomonas aeruginosa sodA andsodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism.J.Bacteriol. 177, 6330–6337 (1995).
Høiby N.: Prospects for the prevention and control of pseudomonal infection in children with cystic fibrosis.Paediatr. Drugs 2, 451–463 (2000).
Kadry A.A.: Lack of efflux mechanism in a clinical isolate ofPseudomonas aeruginosa highly resistant to β-lactams and imipenem.Folia Microbiol. 48, 529–533 (2003).
Karakoç B., Gerçeker A.A.:In vitro activities of various antibiotics, alone and in combination with amikacin againstPseudomonas aeruginosa.Internat.J.Antimicrob.Agents 18, 567–570 (2001).
Köhler T., Van Delden C., Kocjancic Curty L., Hamzehpour M.M., Pechere J.C.: Overexpression of the Mex EF-Opr N multidrug efflux system affects cell-to-cell signaling inPseudomonas aeruginosa.J.Bacteriol. 183, 5213–5222 (2001).
Liu P.V., Abe Y., Bates J.L.: The roles of various fractions ofPseudomonas aeruginosa in its pathogenesis.J.Infect.Dis. 108, 218–228 (1961).
Majtan V., Hoštacká A., Majtánova L’., Trupl J.: Toxinogenicity and markers of pathogenicity ofPseudomonas aeruginosa strains isolated from patients with tumor diseases.Folia Microbiol. 47, 445–449 (2002).
Mathai D., Lewis M.T., Kugler K.C., Pfaller M.A., Jones R.N.,The Sentry Participants Group (North America): Antibacterial activity of 41 antimicrobials tested against over 2773 bacterial isolates from hospitalized patients with pneumonia — I. Results from theSentry Antimicrobial Surveillance Program (North America 1998).Diagn.Microbiol.Infect.Dis. 39, 105–116 (2001).
Okazaki M., Onogawa T., Araki K., Egami T., Furuya N., Endo N., Uchimura H.: Isolation frequency and biological characteristics of the multiple-antibiotic resistantPseudomonas aeruginosa isolated from clinical specimens.Kansen-shogaku Zasshi 71, 1181–1186 (1997).
O’Toole G.A., Kolter R.: Initiation of biofilm formation inPseudomonas fluorescens WCS 365 proceedsvia multiple, convergent signalling pathways: a genetic analysis.Mol.Microbiol. 28, 449–461 (1998a).
O’Toole G.A., Kolter R.: Flagellar and twitching motility are necessary forPseudomonas aeruginosa biofilm development.Mol.Microbiol. 30, 295–304 (1998b).
Paraskaki I., Lebessi E., Legakis N.J.: Epidemiology of community-acquiredPseudomonas aeruginosa infections in children.Eur.J.Clin.Microbiol.Infect.Dis. 15, 782–786 (1996).
Pesci E.C., Iglewski B.H.: Quorum sensing, pp. 55–65 in D.L. Burns, J.T. Barbieri, B.H. Iglewski, R. Rappouli (Eds):Bacterial Protein Toxins. ASM Press, Washington (DC) 2003.
Puzová H., Siegfried L., Kmeťová M., Ďurovičová J., Kerestešová A.: Characteristics ofPseudomonas aerigunosa strains isolated from urinary tract infections.Folia Microbiol. 39, 337–341 (1994).
Ravn L., Christensen A.B., Molin S., Givskov M., Gram L.: Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics.J.Microbiol.Meth. 44, 239–251 (2001).
Rosenberg M., Gutnick D., Rosenberg E.: Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity.FEMS Microbiol.Lett. 9, 29–33 (1980).
Seib K.L., Tseng H.J., McEwan G., Apicella M.A., Jennings M.P.: Defenses against oxidative stress inNeisseria gonorrhoeae andNeisseria meningitidis: distinctive systems for different lifestyles.J.Infect.Dis. 190, 136–147 (2004).
Singh P.K., Schaefer A.L., Parsek M.R., Moninger T.O., Welsh M.J., Greenberg E.P.: Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms.Nature 407, 762–764 (2000).
Struelens M.J., Denis O., Rodriquez-Villalobos H.: Microbiology of nosocomial infections: progress and challenges.Microb.Infect. 6, 1043–1048 (2004).
Van Delden C., Iglewski B.H.: Cell-to-cell signaling andPseudomonas aeraginosa infections.Emerg.Infect.Dis. 4, 551–560 (1998).
Van Houdt R., Aersten A., Jansen A., Quintana A.L., Michiels C.W.: Biofilm formation and cell-to-cell signaling in Gram-negative bacteria isolated from a food processing environment.J.Appl.Microbiol. 96, 177–184 (2004).
Woods D.E., Sokol P.A., Bryan L.E., Storey D.G., Mattingly M.J., Vogel H.J., Ceri H.:In vivo regulation of virulence inPseuaomonas aeruginosa associated with genetic rearrangement.J.Infect.Dis. 163, 143–149 (1991).
Yadav V., Harjai K., Kaur R., Joshi K., Sharma S.: Urovirulence ofPseudomonas acruginosa: planktonic cellsvs. biofilm cells.Folia Microbiol. 49, 465–471 (2004).
Žatkovič B., Trupl J., Majtán V.: Survey of susceptibility to selected antibiotics ofPseudomonas aeruginosa strains isolated from patients with tumor disease and their relationship to serotype.Folia Microbiol. 38, 415–420 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoštacká, A., Čižnár, I., Slobodníková, L. et al. ClinicalPseudomonas aeruginosa: Potential factors of pathogenicity and resistance to antimicrobials. Folia Microbiol 51, 633–638 (2006). https://doi.org/10.1007/BF02931631
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02931631