Abstract
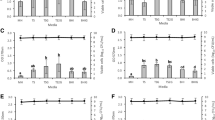
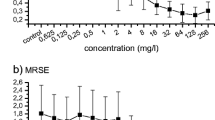
Based on the ability to attach to polymeric surfaces, the formation of biofilms was determined in 5 wild-type strains (Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumanii, Escherichia coli, Staphylococcus warneri). Using modified Christensen method, minimum regrowth concentration (MRC) of piperacillin, piperacillin-tazobactam, cefoperazon, ceftazidim, cefepim, meronem, ciprofloxacin, netilmicin and amikacin for Gram-negative and of ampicillin-sulbactam, chloramphenicol, tetracycline, clindamycin, vancomycin and teicoplanin for Gram-positive bacteria was estimated in trypticase-soy broth medium after a 1-d growth on polystyrene microtiter plates. Adherent bacterial populations exhibited reduced antimicrobial susceptibility, which was not shown in submerged cultures. Our results indicate that MRC can predict therapeutic outcome of antibiotic treatment better than the minimum inhibitory concentration tests commonly used.
Similar content being viewed by others
References
Amorena B., Gracia E., Monzón M., Leiva J., Oteiza C., Pérez M., Alabart J.L., Hernández-Yago J.: Antibiotic susceptibility assay forStaphylococcus aureus in biofilms developedin vitro.J.Antimicrob.Chemother. 44, 43–55 (1999).
Ceri H., Olson M.E., Stremick C., Read R.R., Morck D., Buret A.: The Calgary Biofilm Device: a new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms.J.Clin.Microbiol. 37, 1771–1776 (1999).
Christensen G.D., Simpson W.A., Bisno A.L., Beachey E.H.: Adherence of slime-producing strains ofStaphylococcus epidermidis to smooth surfaces.Infect.Immun. 37, 318–326 (1982).
Christensen G.D., Simpson W.A., Younger J.J., Baddour L.M., Barrett F.F., Melton D.M., Beachey E.D.: Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices.J.Clin.Microbiol. 22, 996–1006 (1985).
Costerton J.W., Stewart P.S., Grenberg E.P.: Bacterial biofilms: a common cause of persistent infections.Science 284, 1318–1322 (1999).
Cramton S.E., Gerke C., Schnell N.F., Nichols W.W., Götz F.: The intercellular adhesion (ica) locus is present inStaphylococcus aureus and is required for biofilm formation.Infect.Immun. 67, 5427–5433 (1999).
Donlan R.M., Murga R., Bell M., Toscano C.M., Carr J.H., Novicki T.J., Zuckerman C., Corey L.C., Miller J.M.: Protocol for detection of biofilms on needleless connectors attached to central venous catheters.J.Clin.Microbiol. 39, 750–753 (2001).
Hoyle B.D., Costerton J.W.: Bacterial resistance to antibiotics: the role of biofilms.Progr.Drug Res. 37, 91–105 (1991).
Knobloch K.M., von Osten H., Horstkotte A., Rohde H., Mack D.: Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negativeStaphylococcus epidermidis.Med.Microbiol.Immunol. 191, 107–114 (2002).
König C., Schwank S., Blaser J.: Factors compromising antibiotic activity against biofilm ofStaphylococcus epidermidis.Eur. J.Clin.Microbiol.Infect.Dis. 20, 20–26 (2001).
McKenney D., Hübner J., Müller E., Wang Y., Goldmann D.A., Pier G.B.: Theica locus ofStaphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin.Infect.Immun. 66, 4711–4720 (1998).
Monzon M., Oteiza C., Leiva J., Amorena B.: Synergy of different antibiotic combinations in biofilms ofStaphylococcus epidermidis.J.Antimicrob.Chemoter. 48, 793–801 (2001).
Lyte M., Freestone P.P.E., Neal C.P., Olson B.A., Haigh R.D., Bayston R., Williams P.H.: Stimulation ofStaphylococcus epidermidis growth and biofilm formation by catecholamine inotropes.Lancet 361, 130–135 (2003).
Shiro H., Müller E., Gutierrez N., Boisot S., Grout M., Tosteson T.D., Goldmann D., Pier G.B.: Transpozon mutants ofStaphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis.J.Infect.Dis. 169, 1042–1049 (1994).
Song W., Woo L., Kim J.S., Lee K.M.:In vitro activity of β-lactams in combination with other antimicrobial agents against resistant strains ofPseudomonas aeruginosa.Internat.J.Antimicrob.Agents 21, 8–12 (2003).
Stewart P.S., Costerton J.W.: Antibiotic resistance of bacteria in biofilms.Lancet 358, 135–138 (2001).
Stone J.H., Gabriel M.M., Ahearn D.G.: Adherence ofPseudomonas aeruginosa to inanimate polymers including biomaterials.J.Ind.Microbiol.Biotechnol. 23, 713–717 (1999).
Tunney M.M., Patrick S., Gorman S.P., Nixon J.R., Anderson N., Davis R.I., Hanna D., Ramage G.: Improved detection of infection in hip replacements.J.Bone Joint Surg. 80B, 568–572 (1998).
Watnick P., Kolter R.: Biofilm, city of microbes.J.Bacteriol. 182, 2675–2679 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grant no. 6818-3 from theGrant Agency of the Ministry of Health of the Czech Republic.
Rights and permissions
About this article
Cite this article
Černohorská, L., Votava, M. Determination of minimal regrowth concentration (MRC) in clinical isolates of various biofilm-forming bacteria. Folia Microbiol 49, 75–78 (2004). https://doi.org/10.1007/BF02931650
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02931650