Abstract
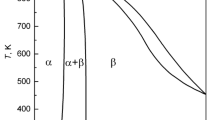
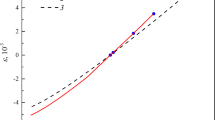
Magnesium activities in liquid magnesium-lead alloys have been measured with electromotive force cells over the temperature range 745 to 868 K and over the mole fraction range 0.03 <X Mg<0.50. The exper imental data were used to evaluate the parameters in the empirical temperature-composition relationship proposed by Krupkowski for describing the activity coefficients. Values generated from the Krupkowski formulae were utilized with data for liquidus compositions to evaluate the free energies of formation of the solid intermediate phases and compared with available experimental data for Mg2Pb.
Similar content being viewed by others
References
A. Krupkowski:Bull. Acad. Pol. Sci. Lett., Ser. A, 1950, vol. 1, pp. 15–45;Bull. Acad. Pol. Sci., Ser. Sci. Techn., 1969, vol. 27, pp. 87–93.
Z. Moser:Met. Trans. B, 1975, vol. 6B, in Press.
Z. Moser:Met Trans., 1975, vol. 6B, pp. 103–09.
Z. Moser and W. Zakulski,J. Electrochem. Soc., 1975, vol. 122, pp. 232–38.
Z. Moser,Met. Trans, 1974, vol. 5, pp. 1445–50.
Z. Moser and K. Fitzner:Proc. Symp. on the Themodynamics of Nuclear Materials, held in Vienna, Octover 21 to 25, 1974, to be published.
Z. Moser and C. Krohn,Met. Trans., 1974, vol. 5, pp. 979–85.
J. M. Eldridge E. Miller, and K. L. Komarek:Trans. TMS-AIME, 1965, vol. 233, pp. 1303–08.
K. K. Kelley:U.S. Bur. Mires, Bull., no. 584, 1960.
D. R. Stull and G. C. Sinke:Themodynamic Properties of the Elements, Amer. Chem. Soc., Washington, D.C., 1965.
R. R. Hultgren, R. L. Orr, and K. K. Kelley:Selected Values of the Thermodynamic Properties of the Elements, ASM, Metals Park, Ohio, 1973.
Z. Moser:Rev. Roum. Chim., 1971, vol. 16, pp. 327–41.
R. A. SharmaJ. Chem. Thermodynamies, 1970, vol. 2, pp. 373–89.
A. Knappwost:Z. Phys. Chem. (Frankfurt an Main), 1959, vol. 21, pp. 358–75.
M. F. Lantratov:Russ. J. Inorg. Chem., vol. 4, 636–38.
I. T. Sryvaiin, O. A. Esin, and B. M. Lepinskikh:Russ. J. Phys. Chem., (Engl. Trans.) 1964, vol. 38, pp. 637–41.
J. M. Eldridge, E. Miller, and K. L. Komarek:Trans. TMS-AIME, 1967, vol. 239, pp. 570–72.
J. R. Wilson: March 1966, Queen's University, Kingston, Ontario, private communication quoted by Eldridgeet al (Ref. 17).Trans. TMS-AIME, 1967, vol. 239, pp. 570-72.
P. Beardmore, B. W. Howlett, B. D. Lichter, and M. B. Bever:Trans. TMS-AIME, 1966, vol. 236, pp. 102–08.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moser, Z., Smith, J.F. Thermodynamic properties of magnesium-lead alloys. Metall Trans B 6, 457–460 (1975). https://doi.org/10.1007/BF02913832
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02913832