Abstract
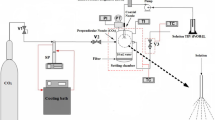
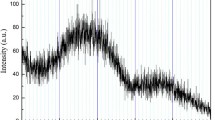
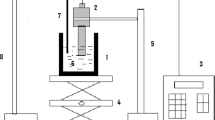
The synthesis of manganese oxide and LiMn2O4 particles in supercritical water has been investigated with a residence time of 10–40 seconds. It was suggested that the reaction temperature for SCW process should be relatively higher than the critical temperature of water, to synthesize the particles of uniform size and shape. It was observed that the selective synthesis of LiMn2O4 was mainly dependent of the amount of OH- ion in the reactants. We concluded that the size, shape and structure of particles were strongly influenced by a change in the reaction temperature, reactant composition and OH- ion amount, and thus enabling to synthesize a specific metal oxide particles. The reaction mechanisms for manganese oxides and LiMn2O4 have been proposed with the oxidation, hydrolysis and dehydration steps.
Similar content being viewed by others
References
Adschiri, T., Kanazawa, K. and Arai, K.,“Rapid and continuous hydrothermal crystallization of metal oxide particles in supercritical water,”J. Am. Ceram. Soc.,75, 1019 (1992).
Adschiri, T., Hakuta, Y. and Arai, K.,“Hydrothermal synthesis of metal oxide fine articles at supercritical conditions,”Ind. Eng. Chem. Res.,39, 4901(2000).
Bailar, J. C., Emeleus, H. J., Nyholm, S. R. and Trotman-Dickenson, A. F.,Comprehensive inorganic chemistry, vol. 3, Pergamon Press, New York (1976).
Cabaoas, A. and Poliakoff, M.,“The continuous hydrothermal synthesis of nano-particulate ferrites in near critical and supercritical water,”J. Mater. Chem.,11, 1408 (2001).
Cotton, F. A., Wilkinson, G., Murillo, C. A. and Bochmann, M.,Advanced inorganic chemistry, 6th Edition, John Wiley & Sons, Inc., New York (1999).
Hakuta, Y., Terayama, H., Onai, S., Adschiri, T. and Arai, K.,“Hydrothermal synthesis of CeO2 fine particles in supercritical water,” Proc. 4th Int. Symp. on ‘Supercritical Fluids,’ Sendai, Japan, May, 255–258 1997.
Hakuta, Y., Adschiri, T., Suzuki, X., Chida, T., Senio, K. and Arai, K., “Flow method for rapidly producing barium hexaferrite particles in supercritical water,”J. Am. Ceram. Soc.,81, 2461 (1998).
Hakuta, Y., Senio, K., Ura, H., Adschiri, T., Takizawa, H. and Arai, K., “Production of phosphor (YAG: Tb) fine particles by hydrothermal synthesis in supercritical water,”J. Mater. Chem.,9, 2671 (1999).
Hakuta, Y., Yamamoto, Y., Okada, K.-I., Adschiri, T., Arai, K., Kanamura, K., Goto, A., Hamagami, J. and Umegaki, T.,“Production of spinel LiMn2O4 fine crystals via hydrothermal synthesis in supercritical water,” Proc. 40th Symp.in Japan on ‘attery,’ Kyoto, Japan, November, 251–252 (1999).
Holleman, A. F. and Wiberg, E.,Inorganic chemistry, 34th Edn, Academic Press, San Diego (2001).
Lee, J.-H., Kim, M.-H., Choo, K.-H. and Ham, J.-Y.,“Synthesis of TiO2 particles in water from subcritical to supercritical conditions,”J. Korean Ind. Eng. Chem.,14, 847 (2003).
Nam, S.-C. and Kim, G.-J.,“Characterization of barium hexaferrite produced by varying the reaction parameters at the mixing-points in a supercritical water crystallization process,”Korean J. Chem. Eng.,21, 582 (2004).
Rho, S.-W. and Park, S.-D.,“Influence of stoichiometry and alkalinity on barium hexaferrite formation via the supercritical water crystallization method,”Korean J. Chem Eng.,19, 120 (2002).
Wakihara, M., Li, G. and Ikuta, H.,Cathode active materials with a threedimensional spinel framework, Lithium Ion Batteries, Wakihara, M. and Yamamoto, O., eds., Wiley-Vch (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JH., Ham, JY. Synthesis of manganese oxide particles in supercritical water. Korean J. Chem. Eng. 23, 714–719 (2006). https://doi.org/10.1007/BF02705916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705916