Abstract
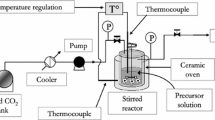
Mesoporous titanium dioxide particles (TiO2) were prepared by reactive precipitation in the supercritical anti-solvent (SAS) technique using carbon dioxide in an ambient saturated with water in the presence of the ionic liquid (IL) 1-methyl-3-octylimidazolium bis[trifluoromethylsulfonyl] imide, [C8mim][NTf2]. All experiments of reactive precipitation with SAS were conducted at 40 °C, with a pressure of 8.0 MPa, a CO2 liquid flow rate of 20 mL min–1, and a solution flow rate of 2 mL min–1. Additionally, the influence of the molar ratio of titanium(IV) isopropoxide/isopropanol and ionic liquid/titanium(IV) isopropoxide was investigated. The particles were characterized by X-ray powder diffraction, thermogravimetric analysis, N2 adsorption-desorption analysis (BET surface area), and field-emission gun scanning electron microscopy. Results indicate the formation of anatase and brookite crystalline phases after calcination at 450 °C for 2 h. Besides, the peaks related to the brookite phase were more intense on samples synthesized using the IL. The synthesized TiO2 particles have suitable structural properties, such as high surface area, controlled porosity, narrow pore size distribution (lower than 6 nm), and high thermal stability. Further, particles with spherical morphology are produced, while those synthesized with the IL present smooth surfaces. The use of the IL decreases the particle size from 1.12 ± 0.78 μm (TiO2 without IL) to particles in the range from 0.32 ± 0.13 to 0.66 ± 0.44 μm. Furthermore, the higher the precursor/alcohol ratio, the larger the particle size, thus demonstrating that the particle size also depends on the precursor/alcohol ratio.
Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Additionally, raw data are available on request from the corresponding author.
References
Aaymonier C et al (2006) Review of supercritical fluids in inorganic materials science. J Supercrit Fluids 38:242–251. https://doi.org/10.1016/j.supflu.2006.03.019
Adschiri T, Yoko A (2018) Supercritical fluids for nanotechnology. J Supercrit Fluids 134:167–175. https://doi.org/10.1016/j.supflu.2017.12.033
Agartan L et al (2015) Effect of initial water content and calcination temperature on photocatalytic properties of TiO2 nanopowders synthesized by the sol–gel process. Ceram Int 41:12788–12797. https://doi.org/10.1016/j.ceramint.2015.06.114
Ahmad MS, Pandey AK, Rahim NA (2017) Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew Sustain Energy Rev 77:89–108. https://doi.org/10.1016/j.rser.2017.03.129
Alonso E et al (2007) Synthesis of titanium oxide particles in supercritical CO2: Effect of operational variables in the characteristics of the final product. J Supercrit Fluids 39:453–461. https://doi.org/10.1016/j.supflu.2006.03.006
Bianchi CL et al (2014) Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: comparison between nano and micro-sized TiO2. Appl Catal B-Environ 146:123–130. https://doi.org/10.1016/j.apcatb.2013.02.047
Blanchard LA et al (1999) Green processing using ionic liquids and CO2. Nature 399:28–29. https://doi.org/10.1038/19887
Blanchard LA et al (2001) High-pressure phase behavior of ionic liquid/CO2 systems. J Phys Chem B 105:2437–2444. https://doi.org/10.1021/jp003309d
Bu X, Zhang G, Zhang C (2012) Effect of nitrogen doping on anatase–rutile phase transformation of TiO2. Appl Surf Sci 258:7997–8001. https://doi.org/10.1016/j.apsusc.2012.04.154
Cansell F, Aymonier C (2009) Design of functional nanostructured materials using supercritical fluids. J Supercrit Fluids 47:508–516. https://doi.org/10.1016/j.supflu.2008.10.002
Chen Y, Mu TC (2021) Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem Eng 2:174–186. https://doi.org/10.1016/j.gce.2021.01.004
Chen PC, Chen CC, Chen SH (2017) A review on production, characterization, and Photocatalytic applications of TiO2 nanoparticles and nanotubes. Curr Nanosci 13:373–393. https://doi.org/10.2174/1573413713666170511163542
Choi H et al (2006) Thermally stable nanocrystalline TiO2 photocatalysts synthesized via sol–gel methods modified with ionic liquid and surfactant molecules. Chem Mater 18:5377–5384. https://doi.org/10.1021/cm0615626
Choi EH, Hong SI, Moon DJ (2008) Preparation of thermally stable mesostructured nano-sized TiO2 particles by modified sol–gel method using ionic liquid. Catal Lett 123:84–89. https://doi.org/10.1007/s10562-008-9398-4
Cui L et al (2012) Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes. Mater Lett 75:175–178. https://doi.org/10.1016/j.matlet.2012.02.004
Dahl M, Liu Y, Yin Y (2014) Composite Titanium Dioxide Nanomaterials. Chem Rev 114:9853–9889. https://doi.org/10.1021/cr400634p
Dong R et al (2014) TiO2 microspheres with variable morphology, size and density synthesized by a facile emulsion-mediated hydrothermal process. Mater Lett 123:135–137. https://doi.org/10.1016/j.matlet.2014.03.008
Franceschi E et al (2008) Phase behavior and process parameters effects on the characteristics of precipitated theophylline using carbon dioxide as antisolvent. J Supercrit Fluid 44:8–20. https://doi.org/10.1016/j.supflu.2007.09.031
Freire MG et al (2008) Mutual solubilities of water and the [Cnmim][Tf2N] hydrophobic ionic liquids. J Phys Chem B 112:1604–1610. https://doi.org/10.1021/jp7097203
Ghows N, Entezari MH (2010) Ultrasound with low intensity assisted the synthesis of nanocrystalline TiO2 without calcination. Ultrason Sonochem 17:878–883. https://doi.org/10.1016/j.ultsonch.2010.03.010
Gunti S, Kumar A, Ram MK (2018) Nanostructured photocatalysis in the visible spectrum for the decontamination of air and water. Int Mater Rev 63:257–282. https://doi.org/10.1080/09506608.2017.1379264
Hu S et al (2008) Synthesis of mesostructure anatase TiO2 particles in room-temperature ionic liquids. Mater Lett 62:2954–2956. https://doi.org/10.1016/j.matlet.2008.01.082
Huddleston JG et al (1998) Room temperature ionic liquids as novel media for clean liquid–liquid extraction. Chem Commun 16:1765–1766. https://doi.org/10.1039/a803999b
Humayun M et al (2018) Modification strategies of TiO2 for potential applications in photocatalysis: a critical review. Green Chem Lett Rev 11:86–102. https://doi.org/10.1080/17518253.2018.1440324
Katal R et al (2020) A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem Eng J 384:123384. https://doi.org/10.1016/j.cej.2019.123384
Keskin et al (2007) A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids 43:150–180. https://doi.org/10.1016/j.supflu.2007.05.013
Larder RR et al (2021) Porous hollow TiO2 microparticles for photocatalysis: exploiting novel ABC triblock terpolymer templates synthesized in supercritical CO2. Polym Chem 12:2904–2913. https://doi.org/10.1039/D1PY00334H
Leyva-Porras C et al (2015) Low-temperature synthesis and characterization of anatase TiO2 nanoparticles by an acid assisted sol–gel method. J Alloys Compd 647:627–636. https://doi.org/10.1016/j.jallcom.2015.06.041
Liu H et al (2009) Hydrothermal synthesis of mesostructured nanocrystalline TiO2 in an ionic liquid–water mixture and its photocatalytic performance. Solid State Sci 11:1655–1660. https://doi.org/10.1016/j.solidstatesciences.2009.06.011
Mamaghani AH, Haghighat F, Lee CS (2019) Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 219:804–825. https://doi.org/10.1016/j.chemosphere.2018.12.029
Marin RP et al (2015) Supercritical antisolvent precipitation of TiO2 with tailored anatase/rutile composition for applications in redox catalysis and photocatalysis. Appl Catal A-Gen 504:62–73. https://doi.org/10.1016/j.apcata.2015.02.023
Miao S et al (2006) Synthesis of mesoporous TiO2 films in ionic liquid dissolving cellulose. Micropor Mesopor Mat 95:26–30. https://doi.org/10.1016/j.micromeso.2006.04.013
Paszkiewicz M et al (2016) The ILs-assisted solvothermal synthesis of TiO2 spheres: the effect of ionic liquids on morphology and photoactivity of TiO2. Appl Catal B-Environ 184:223–237. https://doi.org/10.1016/j.apcatb.2015.11.019
Shahi SK et al (2015) Green synthesis of photoactive nanocrystalline anatase TiO2 in recyclable and recoverable acidic ionic liquid [Bmim] HSO4. J Mater Sci 50:2443–2450. https://doi.org/10.1007/s10853-014-8799-6
Shahi SK, Kaur N, Singh V (2016) Fabrication of phase and morphology controlled pure rutile and rutile/anatase TiO2 nanostructures in functional ionic liquid/water. Appl Surf Sci 360:953–960. https://doi.org/10.1016/j.apsusc.2015.11.092
Shayegan Z, Lee CS, Haghighat F (2018) TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chem Eng J 334:2408–2439. https://doi.org/10.1016/j.cej.2017.09.153
Shen W et al (2015) Preparation of titanium dioxide nanoparticle modified photocatalytic self-cleaning concrete. J Clean Prod 87:762–765. https://doi.org/10.1016/j.jclepro.2014.10.014
Silva EP et al (2014) scCO2-based synthesis of semi-crystalline TiO2 nanoparticles: a rapid and direct strategy. Mater Lett 136:133–137. https://doi.org/10.1016/j.matlet.2014.07.156
Silva EP et al (2019) Effect of phase composition on the photocatalytic activity of titanium dioxide obtained from supercritical antisolvent. J Colloid Interf Sci 535:245–254. https://doi.org/10.1016/j.jcis.2018.09.098
Sing KSW et al (1985) Reporting physisorption data for gas-solid systems. Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198254112201
Stallings WE, Lamb HH (2003) Synthesis of nanostructured titania powders via hydrolysis of titanium isopropoxide in supercritical carbon dioxide. Langmuir 19:2989–2994. https://doi.org/10.1021/la020760i
Sui R, Rizkalla A, Charpentier PA (2011) Experimental study on the morphology and porosity of TiO2 aerogels synthesized in supercritical carbon dioxide. Micropor Mesopor Mat 142:688–695. https://doi.org/10.1016/j.micromeso.2011.01.016
Tadros ME et al (1996) Synthesis of titanium dioxide particles in supercritical CO2. J Supercrit Fluids 9:172–176. https://doi.org/10.1016/S0896-8446(96)90029-7
Tang ZR et al (2006) Preparation of TiO2 using supercritical CO2 antisolvent precipitation (SAS): a support for high activity gold catalysts. Stud Surf Sci Catal 162:219–226. https://doi.org/10.1016/S0167-2991(06)80910-9
Verma YL, Singh MP, Singh RK (2012) Ionic liquid assisted synthesis of nano-porous TiO2 and studies on confined ionic liquid. Mater Lett 86:73–76. https://doi.org/10.1016/j.matlet.2012.07.025
Wu D et al (2015) Tunable synthesis of single-crystalline-like TiO2 mesocrystals and their application as effective scattering layer in dye-sensitized solar cells. J Colloid Interf Sci 456:125–131. https://doi.org/10.1016/j.jcis.2015.06.023
Yoo KS, Lee TG, Kim J (2005) Preparation and characterization of mesoporous TiO2 particles by modified sol–gel method using ionic liquids. Micropor Mesopor Mat 84:211–217. https://doi.org/10.1016/j.micromeso.2005.05.029
Zhang B et al (2015) Ionic liquid-assisted synthesis of morphology controlled TiO2 particles with efficient photocatalytic activity. RSC Adv 5:81108–81114. https://doi.org/10.1039/C5RA17213F
Zhao H, Xia S, Ma P (2005) Use of ionic liquids as ‘green’solvents for extractions. J Chem Technol Biotechnol: Int Res Process Environ Clean Technol 80(10):1089–1096. https://doi.org/10.1002/jctb.1333
Acknowledgements
The authors thank the National Council of Technological and Scientific Development–CNPq (grants 304419/2015-0, 305438/2018-2, 313453/2018-7), the Coordination for the Improvement of Higher Education Personnel–CAPES (Finance Code 001) and the Sergipe State Research and Technological Innovation Foundation (FAPITEC/SE) from Brazil for financial support and scholarships supply for this work. This research used facilities of the Multiuser Centre for Nanotechnology at UFS (CMNano-UFS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prado, L.R., Figueiredo, R.T., Silva, R.S. et al. Reactive precipitation of titanium dioxide particles in supercritical CO2 by SAS technique with an ionic liquid as adjuvant. Braz. J. Chem. Eng. 41, 287–298 (2024). https://doi.org/10.1007/s43153-023-00319-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00319-w