Abstract
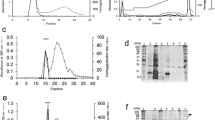
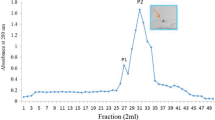
N-Acetyl-D-glucosamine-binding lectin was isolated and purified from rice by ammonium sulphate fractionation and affinity chromatography using N-acetyl-D-glucosamine linked Sepharose 6B column. It gave a single hand on Polyacrylamide disc gel. It was identified as a glycoprotein. The purified lectin dissociated into two components on Sephadex G-100 column chromatography,-a higher molecular weight fraction not containing any carbohydrate and a lower molecular weight glycoprotein fraction. The apparent molecular weights of these fractions were 85,000 and 14,500. The lectin agglutinated erythrocytes of human A,B,O groups and of several other mammals and its activity was inhibited only by N-acetyl-D-glucosamine. The glycopeptide isolated by pronase digestion of the lectin was homogeneous and did not possess agglutinating activity. It contained about 10% carbohydrate of which xylose, arabinose and glucose were the major components.
Similar content being viewed by others
References
Allen, H. J. and Johnson, E. A. Z. (1977)Carbohydr. Res.,58, 253.
Cederburg, B. M. and Gray, G. R. (1979)Anal. Biochem.,99, 221.
Dubois, M., Gilles, K. A., Hamilton, J. K., Robers, P. A. and Smith, F. (1956)Anal. Chem.,28, 350.
Gardell, S. (1953)Acta Chem. Scand.,7, 201.
Goldstein, I. J. and Hayes, C. E. (1978)Adv. Carb. Chem. Biochem.,35, 127.
Kawai, Y. and Anno, K. (1975)Biochem. Biophys. Acta,381, 195.
Kirk, J. E. and Derbye, M. D. (1957)J. Gerontol.,12, 23.
Lis, H., Sela, B., Sachs, L. and Sharon, N. (1970)Biochem. Biophys. Acta.,211, 582.
Lis, H. and Sharon, N. (1972)Methods Enzymol.,B28, 360.
Lis, H. and Sharon, N. (1973)Ann. Rev. Biochem.,42, 541.
Nelson, N. (1944)J. Biol. Chem.,153, 375.
Nicolson, G L. (1974) inInternational review of cytology, eds. G. H. Bourne and J. F. Danielli (New York: Academic Press)39, 89.
Ornstein, L. (1964)Ann. N. Y. Acad. Sci.,121, 321.
Rapin, A. M. C. and Burger, M. M. (1974)Adv. Cancer Res.,20, 1.
Sharon, N. and Lis, H. (1972)Science,177, 949.
Simkin, J. L., Skinner, E. R. and Seshadri, H. S. (1964)Biochem. J.,90, 316.
Somogyi, M. (1952)J. Biol. Chem.,195, 19.
Takahashi, T., Yamada, N., Iwamoto, K., Shimabhayashi, Y. and Izutsu, K. (1973)Agri. Biol. Chem.,37, 29.
Vretblad, P. (1976)Biochim. Biophys. Acta,434, 169.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Indravathamma, P., Seshadri, H.S. Lectin from rice. J. Biosci. 2, 29–36 (1980). https://doi.org/10.1007/BF02703130
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02703130