Abstract
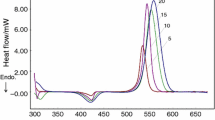
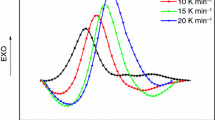
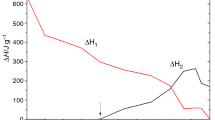
The mechanism and kinetics of curing reaction of tetrafunctional epoxy resin (Ag-80)/novel diamines curing (SED) system were studied by non-isothermal and isothermal DSC. Different equivalent ratios of amine-epoxide give rise to different curing mechanisms. The main condensation reaction can be attributed to the reactions between the primary amine and epoxide and between the hydroxyl and epoxide when temperature is below 200°C, and to the reaction between the second-ary amine and epoxide when temperature is above 200°C. The corresponding apparent activation energies are 58.3 kJ·mol−1 and 99.3 kJ·mol−1 respectively. Apparent activation energies of condensation reactions between primary amine and epoxide and between hydroxyl and epoxide are just the same, which are 47.3 kJ·mol−1.
Zusammenfassung
Mittels nichtisothermer und isothermer DSC wurde der Mechanismus und die Kinetik der Härtungsreaktion von tetrafunktionellem Epoxyharz (Ag-80)/neuartigem Diaminhärtungs(SED) System untersucht. Verschiedene entsprechende Verhältnisse von Amin und Epoxid verursachen verschiedene Härtungsmechanismen. Die wichtigste Kondensationsreaktion kann der Reaktion zwischen primärem Amin und Epoxid und der Reaktion zwischen Hydroxyl und Epoxid zugeschrieben werden, wenn die Temperatur unter 200°C liegt, oberhalb von 200°C hingegen der Reaktion zwischen sekundärem Amin und Epoxid. Die entsprechenden scheinbaren Aktivierungsenergien betragen 58.3 kJ/mol und 99.3 kJ/mol. Die scheinbare Aktivierungsenergie der Kondensationsreaktionen zwischen Amin und Epoxid und zwischen Hydroxyl und Epoxid sind fast gleich, nämlich 47.3 kJ/mol.
Similar content being viewed by others
References
USP 564, 393.
J. Diamant and R. J. Moulton, SAMPE Quarterly, 13 (1984).
USP 4, 645, 803.
P. Delvigs, Polym. Comp., 7 (1986) 101.
Youqing Hua and Huiqiong Wang, 35th Int. SAMPE Symp. and Exhibition, Book 2 of 2 Books, 1990, p. 2035.
Youqing Hua, Huiqiong Wang and Xiping Zhang, 34th Int. SAMPE Symp. and Exhibition, Book 1 of 2 Books, 1989, p. 175.
Youqing Hua and Dongmei Zhao, Journal of Comosite Material (China), 10 (1993) 37
H. E. Kissinger, Anal. Chem., 29 (1957) 1702.
Jiawu Gao, Symposium on Solution Chemistry Thermodynamics, Thermochemistry and Thermal Analysis, Chinese Chemical Society, 1986, p. 291.
N. S. Schneider, J. F. Sprouse and G. L. Hagnauer and J. K. Gillham, Polym. Eng. Sci., 19 (1979) 304.
J. M. Barton, Polymer, 21 (1980) 603.
Leroy Chiao, Macromolecules, 23 (1990) 1286.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hua, Y., Zhao, D. & Quan, X. The mechanism and kinetics of curing reaction of AG-80/SED system by DSC. Journal of Thermal Analysis 45, 177–184 (1995). https://doi.org/10.1007/BF02548677
Issue Date:
DOI: https://doi.org/10.1007/BF02548677