Abstract
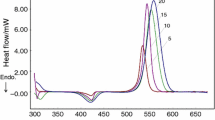
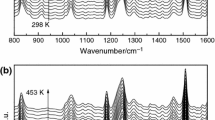
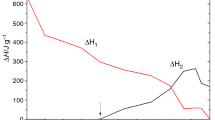
The curing behavior and kinetics of epoxy resin (diglycidyl ether of bisphenol-A) with curing agent polyamidoamine and cyclohexene oxide as active diluents were investigated by differential scanning calorimetry. The apparent activation energy obtained by Kissinger method was 44.37 kJ mol−1. The result of model-fitting kinetic analysis showed that autocatalytic model (Šesták–Berggren model) was suitable to describe the curing mechanism. The thermal–mechanical properties were characterized with dynamic mechanical analysis, and the activation energies for α relaxation were calculated according to the Arrhenius law. Thermogravimetric analysis showed that the performance of the thermal decomposition almost stayed the same although reactive diluent (cyclohexene oxide) was added in curing system.












Similar content being viewed by others
References
Lee SB, Lee HJ, Hong IK. Diluent filler particle size effect for thermal stability of epoxy type resin. J Ind Eng Chem. 2012;18:635–41.
Ratna D, Banthia AK. Rubber toughened epoxy. Macromol Res. 2004;12:11–21.
Zhou HS, Song XX, Xu SA. Mechanical and thermal properties of novel rubber-toughened epoxy blend prepared by in situ pre-crosslinking. J Appl Polym Sci. 2014;131:41110.
Rimdusit S, Jongvisuttisun P, Jubsilp C, Tanthapanichakoon W. Highly processable ternary systems based on benzoxazine, epoxy, and phenolic resins for carbon fiber composite processing. J Appl Polym Sci. 2009;111:1225–34.
Ye Y, Chen H, Wu J, Chan CM. Evaluation on the thermal and mechanical properties of HNT-toughened epoxy/carbon fibre composites. Compos Part B Eng. 2011;42:2145–50.
Chen ZK, Yang G, Yang JP, Fu SY, Ye L, Huang YG. Simultaneously increasing cryogenic strength, ductility and impact resistance of epoxy resins modified by butyl glycidyl ether. Polymer. 2009;50:1316–23.
Liu Y, Yang J-P, Xiao HM, Qu C-B, Feng QP, Fu SY, et al. Role of matrix modification on interlaminar shear strength of glass fibre/epoxy composites. Compos Part B Eng. 2012;43:95–8.
Thakkar J, Patel R, Patel V. Kinetic study on the effect of addition of epoxy diluents and/or fortifier on the curing characteristics of DGEBA by differential scanning calorimetry. Eur Polym J. 1987;23:799–802.
Liu MH, Li C, Liu ZY. Performance of epoxy resin/polyamide-amine curing agent system using epoxy cyclohexene as reactive diluents. J Cent South Univ Sci Technol. 2013;44:520–5.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ghaemy M, Barghamadi M, Behmadi H. Cure kinetics of epoxy resin and aromatic diamines. J Appl Polym Sci. 2004;94:1049–56.
Roşu D, Caşcaval C, Mustatǎ F, Ciobanu C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim Acta. 2002;383:119–27.
Fan M, Li X, Zhang J, Cheng J. Curing kinetics and shape-memory behavior of an intrinsically toughened epoxy resin system. J Therm Anal Calorim. 2015;119:537–46.
Li C, Liu MH, Liu ZY, Qing ML, Wang G. DSC and curing kinetics of epoxy resin using cyclohexanediol diglycidyl ether as active diluents. J Therm Anal Calorim. 2014;116:411–6.
Cheng YY, Chen DZ, Fu RQ, He PS. Behavior of polyamidoamine dendrimers as curing agents in bis-phenol A epoxy resin systems. Polym Int. 2005;54:495–9.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Sun J, Wang J. Study on the optimum ratio and non-isothermal curing kinetics of EP2008/EP2008-S system. J Therm Anal Calorim. 2014;118:571–8.
Senum G, Yang R. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Li C, Fan H, Hu J, Li B. Novel silicone aliphatic amine curing agent for epoxy resin: 1, 3-Bis (2-aminoethylaminomethyl) tetramethyldisiloxane. 2. Isothermal cure, and dynamic mechanical property. Thermochim Acta. 2012;549:132–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M.H., Hou, H.Y., Peng, H.J. et al. The influence of the active diluents cyclohexene oxide on the curing processing. J Therm Anal Calorim 122, 509–515 (2015). https://doi.org/10.1007/s10973-015-4726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4726-6