Summary
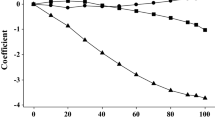
The partial molar free energies at infinite dilution are determined at 25°C by using gas-liquid chromatography. The stationary phase liquids are benzene, toluene, chlorobenzene, benzonitrile, N,N-dimethylacetamide, N,N-dimethylformamide, and dimethyl sulfoxide. The solutes are n-alkanes of ethane to n-heptane, ethyl chloride, bromide and iodide, and propionitrile. When the solvent becomes polar, the values for the n-alkane solutes are found to increase, that for the nitrile to decrease, and those for the halides to behave midway. Empirical analyses of the values of the free energies are presented by comparing the results with those in n-hexane.
Similar content being viewed by others
References
E. F. Meyer, J. Chem. Edu.50, 191 (1973).
J. R. Conder, C. L. Young, “Physicochemical Measurement by Gas Chromatography”, Wiley, New York (1979), Chapters 2 and 3.
S. Terasawa, H. Itsuki, H. Yamaki, Anal. Chem.58, 3021 (1986).
H. Itsuki, S. Terasawa, N. Yamana, S. Ohotaka, Anal. Chem.59, 2918 (1987).
W. Gerrard, J. Appl. Chem. Biotechnol.23, 1 (1973) and results compiled there, but those for butane solute are not included because of too high solubilities at 1 atm.
J. A. Gerster, J. A. Gorton, R.-B. Eklund, J. Chem. Eng. Data5, 423 (1960).
J.-P. Monfort, J. Vidal, H. Renon, J. Chim. Phys.67, 748 (1970).
C. H. Deal, E. L. Derr, Ind. Eng. Chem. Proc. Res. Dev.3, 394 (1964).
L. Rohrschneider, Anal. Chem.45, 1241 (1973).
M. H. Abraham, P. L. Grellier, J. Chem. Soc. Perkin2 1976, 1735.
H. Itsuki, K. Yamashita, S. Terasawa, J. Chem. Thermodynamics20, 1411 (1988).
A. L. McClellan, “Tables of Experimental Dipole Moments”, Freeman, San Francisco and London (1963).
A. Bondi, “Physical Properties of Molecular Crystals, Liquids and Glasses”, Wiley, New York, (1968).
A. B. Littlewood, Anal. Chem.36, 1441 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Itsuki, H., Shigeta, H. & Terasawa, S. GLC determination of partial molar free energies at infinite dilution for simple organic solutes in moderately volatile solvents. Chromatographia 27, 359–363 (1989). https://doi.org/10.1007/BF02321283
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02321283