Abstract
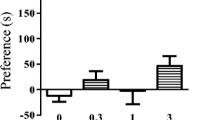
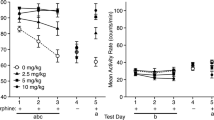
This study sought to determine whether the place learning deficits produced by diazepam are a secondary result of opioid release. Rats pretreated with diazepam (3 mg/kg) or morphine (15 mg/kg) were trained in the Morris water maze. Diazepam impaired place learning-slowing acquisition and preventing the formation of a quadrant preference. Morphine also slowed acquisition, but did not prevent place learning, and impaired escape to a visible platform. Flumazenil blocked the deficits produced by diazepam, but not morphine. Naloxone (2 mg/kg) blocked the deficits produced by morphine, but not diazepam. A high dose of naloxone (10 mg/kg) slowed acquisition, and exacerbated the deficit produced by diazepam. These results demonstrate that diazepam interferes with mnemonic processes through endogenous benzodiazepine receptors, independently of opioidergic systems. Further, they suggest that morphine interferes with motivational processes through opioidergic systems, independently of endogenous benzodiazepine systems.
Similar content being viewed by others
References
Arolfo MP, Brioni JD (1991) Diazepam impairs place learning in the Morris water maze. Behav Neural Biol 55:131–136
Barta A, Yashpal K (1981) Regional redistribution of β-endorphin in the rat brain: The effect of stress. Prog Neuro Psychopharmacol 5:595–598
Billingsley ML, Kubena RK (1978) The effects of naloxone and picrotoxin on the sedative and anticonflict effects of benzodiazepines. Life Sci 22:897–906
Canli TC, Cook RG, Miczek KA (1990) Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacol Biochem Behav 36:521–525
Chang KJ, Hazum E, Cuatrecasas P (1980) Multiple opiate receptors. TINS 3:160–162
Chapman CR, Feather BW (1973) Effects of diazepam on human pain tolerance and pain sensitivity. Psychosom Med 35:330–340
Cole SO (1986) Effects of benzodiazepines on acquisition and performance: a critical assessment. Neurosci Biobehav Rev 10:265–272
Cooper SJ (1983) Benzodiazepine-opiate antagonist interactions in relation to feeding and drinking behavior. Life Sci 32:1043–1051
Decker MW, Introini-Collison IB, McGaugh JL (1989) Effects of naloxone on Morris water maze learning in the rat: enhanced acquisition with pretraining but not posttraining administration. Psychobiology 17:270–275
Dingledine R, Iversen LL, Breuker E (1978) Naloxone as a GABA antagonist: evidence from iontophoretic, receptor binding and convulsant studies. Eur J Pharmacol 47:19–27
Duka T, Wuster M, Herz A (1979) Rapid changes in enkephalin levels in rat striatum and hypothalamus induced by diazepam. Naunyn-Schmiedeberg's Arch Pharmacol 309:1–5
Duka T, Wuster M, Herz A (1980) Benzodiazepines modulate striatal enkephalin levels via a GABAergic mechanism. Life Sci 26:771–776
Duka T, Cumin R, Haefely W, Herz A (1981) Naloxone blocks the effect of diazepam and meprobamate on conflict behavior in rats. Pharmacol Biochem Behav 15:115–117
Duka T, Millan MJ, Ulsamer B, Doenicke E (1982) Naloxone attenuates the anxiolytic action of diazepam in man. Life Sci 31:1833–1836
Ghoneim MM, Mewaldt SP (1990) Benzodiazepines and human memory: a review. Anestesiology 72:926–938
Harsing LG, Yang HYT, Costa E (1982) Evidence for a γ-aminobutyric acid (GABA) mediation in the benzodiazepine inhibition of the release of met5-enkephalin elicited by depolarization. J Pharmacol Exp Ther 220:616–620
Houser VP, Pare WP (1973) Analgesic potency of sodium salicylate, indomethacine, and chlordiazepoxide as measured by a spatial preference technique in the rat. Psychopharmacology 32:121–131
Kuriyama K, Yoneda Y (1978) Morphine induced alterations of γ-aminobutyric acid and taurine contents andl-glutamate decarboxylase activity in rat spinal cord and thalamus: possible correlates with analgesic action of morphine. Brain Res 148:163–179
Lal H, Kumar B, Forester MJ (1988) Enhancement of learning and memory in mice by a benzodiazepine antagonist. FASEB J 2:2707–2711
Lista A, Blier P, De Montigny C (1989) In vivo presynaptic modulation of serotonergic neurotransmission in the rat hippocampus by diazepam. Eur J Pharmacol 171:229–231
Lopez F, Miller LG, Thompson ML, Schatzki A, Chesley S, Greenblatt DJ, Shader RI (1990) Chronic morphine administration augments benzodiazepine binding and GABAA receptor function. Psychopharmacology 101:545–549
McNamara RK, Skelton RW (1991a) Diazepam impairs acquisition but not performance in the Morris water maze. Pharmacol Biochem Behav 38:651–658
McNamara RK, Skelton RW (1991b) Pretraining morphine impairs acquisition and performance in the Morris water maze. Motivation reduction rather than amnesia. Psychobiology 19:313–322
McNamara RK, Whishaw IQ (1990) Blockade of hoarding in rats by diazepam: An analysis of the anxiety and object value hypothesis of hoarding. Psychopharmacology 101:214–221
Miller LG, Kastin AJ, Greenblatt DJ (1987) Tyr-MIF-1 augments benzodiazepine receptor binding in vivo. Pharmacol Biochem Behav 28:521–524
Moroni F, Cheney DL, Peralta E, Costa E (1978) Opiate receptor agonists as modulators of γ-aminobutyric acid turnover in the nucleus caudate, globus pallidus and substantia nigra of the rat. J Pharmacol Exp Ther 207:870–877
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12:239–260
Morris RGM (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Method 11:47–60
Paterson SJ, Robson LE, Kosterlitz HW (1983) Classification of opioid receptors. Br Med Bull 39:31–36
Raffalli-Sebille MJ, Chapouthier G (1991) Similar effects of a beta-carboline and of flumazenil in negatively and positively reinforced learning tasks in mice. Life Sci 48:685–692
Sansone M, Vetulani J (1988) Effect of naloxone on the locomotor stimulatory action of chlordiazepoxide in mice. Pharmacol Biochem Behav 31:371–373
Sarter M, Bruno JP, Dudchenko P (1990) Activating the damaged basal forebrain cholinergic system: tonic stimulation versus signal amplification. Psychopharmacology 101:1–17
Smith JE, Co C, Lane JD (1984) Limbic muscarinic cholinergic and benzodiazepine receptor changes with chronic intravenous morphine and self-administration. Pharmacol Biochem Behav 20:443–450
Soubrie P, Jobert A, Thiebot MH (1980) Differential effects of naloxone against the diazepam-induced release of behavior in rats in three aversive situations. Psychopharmacology 69:101–105
Stapleton JM, Lind MD, Merriman VJ, Reid LD (1979) Naloxone inhibits diazepam-induced feeding in rats. Life Sci 24:2421–2426
Stryker TD, Conlin T, Reichlin S (1986) Influence of a benzodiazepine, midazolam, and gamma-aminobutyric acid (GABA) on basal somatostatin secretion from cerebral and diencephalic neurons in dispersed cell culture. Brain Res 362:339–343
Tripp G, McNaughton N (1987) Naloxone fails to block the effects of chlordiazepoxide on acquisition and performance of successive discrimination. Psychopharmacology 91:119–121
Tripp G, McNaughton N (1991) Naloxone and chlordiazepoxide: effects on acquisition and performance of signalled punishment. Pharmacol Biochem Behav 38:43–47
Tripp G, McNaughton N, Oei TPS (1987) Naloxone blocks the effects of chlordiazepoxide on acquisition but not performance of a differential reinforcement of low rates of response (DRL). Psychopharmacology 91:112–118
Wolkowitz OM, Weingartner H, Thompson K, Pickard D, Paul SM, Hommer DW (1987) Diazepam-induced amnesia: a neuropharmacological model of an “organic amnestic syndrome”. Am J Psychiatry 144:25–29
Wuster M, Duka T, Herz A (1980a) Diazepam-induced release of opioid activity in the rat brain. Neurosci Lett 16:335–337
Wuster M, Duka T, Herz A (1980b) Diazepam effects on striatal met-enkephalin levels following long-term pharmacological manipulations. Neuropharmacology 19:501–505
Yang X, Lou Z, Zhou J (1988) Behavioral evidence for the role of noradrenaline in putative anxiolytic and sedative effects of benzodiazepines. Psychopharmacology 95:280–286
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McNamara, R.K., Skelton, R.W. Pharmacological dissociation between the spatial learning deficits produced by morphine and diazepam. Psychopharmacology 108, 147–152 (1992). https://doi.org/10.1007/BF02245300
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245300