Abstract
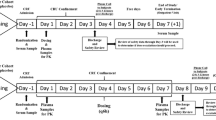
The pharmacokinetics of ceftizoxime, a new β-lactam antibiotic, were studied in eight normal volunteers after single and multiple i.v. doses of 2×2 g daily for eight days. The mean maximum serum concentration after the first 20 min of infusion on the first day was 176.9 ± 32.9 μg/ml decreasing to 11.0 ± 4.1 μg/ml after 4 h and to 0.67 ± 0.2 μg/ml after 12 h. Mean 12 h urine recovery was 78.8 ± 9.0 %. The half-life of ceftizoxime was 136 ± 36 min. Volume of distribution was 22.9 ± 8.1 1/100 kg body weight, AUCtot 208 ± 33 μg/ml · h, total body clearance 151 ± 33 ml/min, and renal clearance 110 ± 23 ml/min. No ceftizoxime accumulation was registered during the administration period. Computed multiple-dose values were in good agreement with the measured values, and the pharmacokinetic parameters on the first, fourth and eighth days showed no significant differences. During the study period, the fecal flora displayed a reduction of sensitive gram-negative aerobic bacteria, a slight increase in the amount of enterococci (106 to 108/g feces), and no change in the number ofBacteroides fragilis. Some resistant gram-negative strains increased during the administration period, but most of them could no longer be detected two weeks after administration. Tolerance of ceftizoxime was good in four volunteers. Four other volunteers had gastro-intestinal symptoms and mild fever reactions. One female volunteer was assumed to have an allergic drug reaction. Biochemical, hematological, virological and serological data showed no abnormalities in seven volunteers.
Similar content being viewed by others
References
Eley, A., Greenwood, D.: In vitro activity of ceftizoxime againstBacteroides fragilis: comparison with benzylpenicillin, cephalothin, and cefoxitin. Antimicrobial Agents and Chemotherapy 1981, 20: 332–335.
Fu, K. P., Neu, H. C.: Antibacterial activity of ceftizoxime, a β-lactamase stable cephalosporin. Antimicrobial Agents and Chemotherapy 1980, 17: 583–590.
Shigi, Y., Kojo, H., Wakasuji, Nishida, M.: Differences between ceftizoxime and its stereoisomer in antibacterial activity and affinity for penicillinbinding proteins. Antimicrobial Agents and Chemotherapy 1981, 19: 393–396.
Tahata, N., Suginaka, H., Kotani, S., Ogawa, M., Kosaki, G.: β-lactam resistance inSerratia marcescens: comparison of action of benzylpenicillin, apalcillin, cefazolin, and ceftizoxime. Antimicrobial Agents and Chemotherapy 1981, 19: 397–401.
Yabuuchi, E., Ito, T., Tanimura, E., Yamamoto, E., Okyama, A.: In vitro antimicrobial activity of ceftizoxime against glucose-non-fermentative gram-negative rods. Antimicrobial Agents and Chemotherapy 1981, 20: 136–139.
Bennett, J. V., Brodie, J. L., Brenner, E. J., Kirby, W. M. M.: Simplified accurate method for antibiotic assay of clinical specimens. Applied Microbiology 1966, 14: 170–178.
Reeves, D. S., Bywater, M. F.: Assay of antimicrobial agents. In: de Louovis, J. (ed.): Selected topics in clinical bacteriology. Bailliére Tindall, London, 1976, p. 21–78.
Lode, H., Kemmerich, B., Koeppe, P.: Comparative clinical pharmacology of gentamicin, sisomicin, and tobramycin. Antimicrobial Agents and Chemotherapy 1975, 8: 396–401.
Neu, M. C., Subramariam, S.: Pharmacology of ceftizoxime compared with that of cefamandole. Antimicrobial Agents and Chemotherapy 1981, 20: 366–369.
Nahashima, N., Suzuki, U., Hashimoto, H., Nishijima, K.: Phase I study of ceftizoxime, a new cephalosporin. Single-dose study. Journal of Clinical Pharmacology 1981, 21: 388–395.
Finegold, S. M., Posnick, P. J., Miller, L. G., Hewitt, W. L.: The effect of various antibacterial compounds on the normal human fecal flora. Ernährungsforschung 1965, 10: 316–341.
Ritschel, W. A.: Handbook of basic pharmacokinetics. Drug Intelligence Publications, Hamilton, 1980, pp. 158–295.
Wagner, J. G.: Biopharmaceutics and relevant pharmacokinetics. Drug Intelligence Publications, Hamilton, 1971, pp. 237–296.
Koeppe, P., Hamann, C.: A program for nonlinear regression analysis to be used on desk-top computers. Computer Programs in Biomedicine 1980, 12: 121–128.
Loo, J. C. K., Riegelmann, S.: Assessment of pharmacokinetic constants from post-infusion blood curves obtained after i.v. infusion. Journal of Pharmacological Science 1970, 59: 53–57.
Greenblatt, D. F., Koch-Weser, J.: Clinical pharmacokinetics. New England Journal of Medicine 1975, 293: 702–705.
Lüthy, R., Blaser, J., Bonetti, A., Siegenthaler, W.: Comparative multiple dose pharmacokinetics of cefotaxime, moxalactam and ceftazidime. Antimicrobial Agents and Chemotherapy 1981, 20: 567–572.
Datta, N., Faiers, M. C., Reeves, D. C., Brumfitt, W., Orskov, F., Orskov, I.: R factors inEscherichia coli in faeces after oral chemotherapy in general practice. Lancet 1971, i: 313–315.
Hartley, C. L., Clements, H. M., Linton, H. B.: Effects of cephalothin, erythromycin and clindamycin on the aerobic gram-negative faecal flora in man. Journal of Medical Microbiology 1978, 11: 125–135.
Knothe, H.: Eigenschaften der Chemotherapeutika und deren Einfluß auf die körpereigene Flora. Wochenschrift der Kinderheilkunde 1977, 125: 262–267.
Schroeter, G., Seeliger, H. P. R., Knothe, H.: Der Einfluß der Antibiotika auf die Darmflora des Menschen. Zentralblatt für Bakteriologie, Parasitenkunde, Infektion und Hygiene 1970, 219: 893–936.
Schimpff, S. C., Young, V. M., Greene, W. H., Vermeulen, G. D., Moody, M. R., Wiesnik, M. D.: Origin of infection in acute nonlymphatic leukemia. Significance of hospital acquisition of potential pathogens. Annals of Internal Medicine 1972, 77: 707–714.
Harnoß, C. M., Fangmann, B., Lode, H., Wagner, J.: Sepsis — Aktuelle Aspekte zur Ätiologie, Klinik und Prognose bei 220 Patienten. Münchner Medizinische Wochenschrift 1982, 124: 504–506. 506.
Sanders, W. E., Johnson, J. E., Taggart, J. G.: Adverse reactions to cephalothin and cephapirin. Uniform occurrence on prolonged intravenous administration of high doses. New England Journal of Medicine 1974, 290: 424–427.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lode, H., Warns, H., Kemmerich, B. et al. Pharmacokinetics of multiple doses of ceftizoxime and their influence on fecal flora. Eur. J, Clin. Microbiol. 2, 116–121 (1983). https://doi.org/10.1007/BF02001576
Issue Date:
DOI: https://doi.org/10.1007/BF02001576