Abstract
Background and Objective
Avibactam is a novel non-β-lactam β-lactamase inhibitor effective against Ambler class A, C and some class D β-lactamases that is currently in clinical development in combination with ceftazidime for the treatment of serious Gram-negative infections. It restores the in vitro activity of a range of β-lactams, including ceftazidime, against extended-spectrum β-lactamase-producing pathogens. Two phase I studies assessed the safety and pharmacokinetics of avibactam in healthy subjects when administered alone or with ceftazidime.
Methods
The first study (NXL104-1001) was a placebo-controlled, single-ascending dose study assessing avibactam 50, 100, 250, 500, 1000, 1500 or 2000 mg given as a 30-min intravenous infusion. After a 7-day washout, subjects in the 250 and 500 mg dosing groups received a second avibactam dose with concomitant ceftazidime 1000 or 2000 mg, respectively. The second study (NXL104-1002) was performed in two parts. Part 1 assessed multiple-ascending doses of avibactam. Subjects were randomized to receive avibactam 500, 750 or 1000 mg every 8 h (q8 h) over 5 days, or ceftazidime-avibactam 2000–500 mg q8 h over 10 days. Part 2 assessed bioavailability of avibactam after a single oral dose (500 mg) relative to a single 30-min intravenous infusion (500 mg).
Results
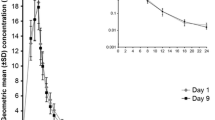
No serious or severe adverse events were reported in either study. Avibactam exposure generally increased proportionally to dose and there was no trend for accumulation after multiple doses. Almost all avibactam was excreted largely unchanged in the urine within the first 6 h. Concomitant ceftazidime did not affect avibactam’s safety and pharmacokinetic profile. Avibactam exposure after oral dosing was very low at 6.2 % of that observed after intravenous infusion.
Conclusion
Avibactam was generally well tolerated across all dosing regimens, when given alone or with ceftazidime. Avibactam exposure was dose related in both studies, and avibactam pharmacokinetics were linear and not affected by ceftazidime.




Similar content being viewed by others
References
Ho J, Tambyah P, Paterson D. Multiresistant Gram-negative infections: a global perspective. Curr Opin Infect Dis. 2010;23:546–53.
Kanj S, Kanafani Z. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250–9.
Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27:128–42.
Peleg A, Hooper D. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–13.
Paterson D. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control. 2006;34:S20–8.
Magiorakos A, Suetens C, Monnet D, et al. The rise of carbapenem resistance in Europe: just the tip of the iceberg? Antimicrob Resist Infect Control. 2013;2:6.
Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8.
Tzouvelekis L, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707.
Stachyra T, Pechereau M, Bruneau J, et al. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother. 2010;54:5132–8.
Aktas Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012;39:86–9.
Lagace-Wiens P, Tailor F, Simner P, et al. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother. 2011;55:2434–7.
Livermore D, Mushtaq S, Warner M, et al. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum beta-lactamases and carbapenemases. J Antimicrob Chemother. 2008;62:1053–6.
Stachyra T, Levasseur P, Pechereau M, et al. In vitro activity of the beta-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother. 2009;64:326–9.
Castanheira M, Sader H, Farrell D, et al. Activity of ceftaroline-avibactam tested against Gram-negative organism populations, including strains expressing one or more beta-lactamases and methicillin-resistant Staphylococcus aureus carrying various staphylococcal cassette chromosome mec types. Antimicrob Agents Chemother. 2012;56:4779–85.
Mushtaq S, Warner M, Williams G, et al. Activity of chequerboard combinations of ceftaroline and NXL104 versus beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2010;65:1428–32.
Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–4.
Crandon J, Schuck V, Banevicius M, et al. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:6137–46.
Endimiani A, Hujer K, Hujer A, et al. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55:82–5.
Housman S, Crandon J, Nichols W, Nicolau D. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother. 2014;58:1365–71.
Miossec C, Merdjan H, Hodgson J. Safety and toxicokinetics of NXL104, a broad spectrum β-lactamase inhibitor, in the rat. In: 45th Interscience conference on antimicrobial agents and chemotherapy. Washington, DC; 2005 (Abstract F-1461).
Association of the British Pharmaceutical Industry. Guidelines for Phase I Clinical Trials. 2007. http://www.abpi.org.uk/our-work/library/reports-surveys/Documents/phase1-trial-guidelines.pdf. Accessed 11 Mar 2015.
Edeki T, Armstrong J, Li J. Pharmacokinetics of avibactam (AVI) and ceftazidime (CAZ) following separate or combined administration in healthy volunteers. In: 53rd Interscience conference on antimicrobial agents and chemotherapy. Denver; 2013 (Abstract A-1019).
Li J, Armstrong J, Edeki T. Pharmacokinetic (PK) drug interaction study of ceftazidime-avibactam (CAZ-AVI) and metronidazole (MTZ) in healthy volunteers. In: 53rd Interscience conference on antimicrobial agents and chemotherapy. Denver; 2013 (Abstract A-1020).
Tarral A, Merdjan H. Effect of age and sex on the pharmacokinetics and safety of avibactam in healthy volunteers. Clin Ther. 2015. doi:10.1016/j.clinthera.2015.01.009 [Epub ahead of print].
Lucasti C, Popescu I, Ramesh M, et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother. 2013;68:1183–92.
Vazquez J, Gonzalez Patzan L, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28:1921–31.
Rains C, Bryson H, Peters D. Ceftazidime. An update of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1995;49:577–617.
Richards D, Brogden R. Ceftazidime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985;29:105–61.
Merdjan H, Tarral A, Haazen W, Evene E, Robertson M, Sable C. Pharmacokinetics and tolerability of NXL104 in normal subjects and patients with varying degrees of renal insufficiency. Clin Microbiol Infect. 2010;16(Suppl 2):S465.
Vishwanathan K, Mair S, Gupta A, et al. Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug Metab Dispos. 2014;42:932–42.
Leroy A, Leguy F, Borsa F, et al. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother. 1984;25:638–42.
Welage L, Schultz R, Schentag J. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob Agents Chemother. 1984;25:201–4.
Li J, Zhou D, Nichols W, Das S. Evaluation of ceftazidime-avibactam (CAZ-AVI) dose regimens for Phase III study in patients with different renal function. In: 52nd Interscience conference on antimicrobial agents and chemotherapy. San Francisco; 2012 (Abstract A-635).
Li J, Knebel W, Riggs M, et al. Population pharmacokinetic modeling of ceftazidime (CAZ) and avibactam (AVI) in healthy volunteers and patients with complicated intra-abdominal infection (cIAI). In: 52nd Interscience conference of antimicrobial agents and chemotherapy. San Francisco; 2012 (Abstract A-634).
Wockhardt UK Ltd. Summary of product characteristics: ceftazidime. 2014. http://www.medicines.org.uk/emc/medicine/21129. Accessed 11 Mar 2015.
Acknowledgments
The authors wish to thank Peter J Laud contracted to AstraZeneca from the Statistical Services Unit, Sheffield, for his expert review of the manuscript. These studies have been presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy in 2007, and were funded by Novexel. Ceftazidime-avibactam is now being developed by AstraZeneca and Forest Laboratories Inc. a subsidiary of Actavis plc. Medical writing support was provided by Catherine Savage and Rob Campbell of Prime Medica Ltd, Knutsford, Cheshire, UK, funded by AstraZeneca. The design and conduct of the study, as well as analysis of the study data and opinions, conclusions, and interpretation of the data, are the responsibility of the authors.
Conflict of interest
Antoine Tarral, Henri Merdjan and Manickam Rangaraju were employees of Novexel SA at the time of conduct of the studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors were formerly employees of Novexel SA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Merdjan, H., Rangaraju, M. & Tarral, A. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Avibactam Alone and in Combination with Ceftazidime in Healthy Male Volunteers: Results of Two Randomized, Placebo-Controlled Studies. Clin Drug Investig 35, 307–317 (2015). https://doi.org/10.1007/s40261-015-0283-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0283-9