Abstract
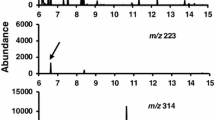
The interaction of obidoxime (Toxogonin®) with sarin was shown by different analytical methods. The UV spectrum of obidoxime at pH 7.4 yields two absorption maxima, λ1=284 nm and λ2=353 nm. The peak at λ2=353 nm is representative for the amount of zwitterionic obidoxime, i.e. the active form of obidoxime. By addition of sarin, λ1 shifts immediately to 278 nm and the intensity at λ2 decreases, thus indicating an interaction. TLC and31P-NMR evidence shows that both mono-phosphonylated and diphosphonylated obidoximes are present. Decomposition of phosphonylated obidoxime in MOPS (3-[N-morpholino] propanesulfonic acid) buffered D2O at pH 7.4 occurs with t1/2=13.3 min at 24°C. Decomposition of di-phosphonylated obidoxime is faster. It is suggested that decomposition of di-phosphonylated obidoxime occurs through the mono-phosphonylated form. Formation and decomposition of mono- and di-phosphonylated obidoxime is pH dependent. We conclude that obidoxime exerts a detoxifying effect by capturing free sarin molecules and thus increasing its polarity. Thereby the transition of sarin through the blood-brain barrier is restricted and its renal elimination facilitated.
Similar content being viewed by others
References
Christenson I (1968a) Hydrolysis of bis (4-hydroxyiminomethyl-1-pyridiniomethyl)ether dichloride (Toxogonin®): I. Decomposition products. Acta Pharm Suecica 5: 23–36
Christenson I (1968b) Hydrolysis of bis (4-hydroxyiminomethyl-1-pyridiniomethyl)ether dichloride (Toxogonin®): II. Kinetics and equilibrium in acidic solution. Acta Pharm Suecica 5: 249–262
Christenson I (1972) Hydrolysis of obidoxime chloride (Toxogonin®): III. Kinetics in neutral and alkaline solution. Acta Pharm Suecica 9: 309–322
Erdmann WD, Engelhard H (1964) Pharmakologisch-toxikologische Untersuchungen mit dem Dichlorid des Bis-[4-hydroxyimino-methyl-pyridinium-(1)-methyl-äthers, einem neuen Esterase Reaktivator. Drug Res 14: 5–11
Eyer P, Hell W, Kawan A, Klehr H (1986) Studies on the decomposition of the oxime HI 6 in aqueous solution. Arch Toxicol 59: 266–271
Eyer P, Ladstetter B, Schäfer W, Sonnenbichler J (1989) Studies on the stability and decomposition of the Hagedorn-oxime HLö 7 in aqueous solution. Arch Toxicol 63: 59–67
Green AL, Saville B (1956) The reaction of oximes with isopropyl methylphosphonofluoridate (sarin). J Chem Soc 27: 3887–3892
Hackley BE, Steinberg GM, Lamb JC (1959) Formation of potent inhibitors of AChE by reaction of pyridinealdoximes with isopropyl methylphosphonofluoridate (GB). Biochem Biophys Acta 80: 211–214
Hagedorn I, Gündel WH, Schoene K (1968) Reactivierung phosphorylierter Acetylcholin-Esterase mit Oximen: Beitrag zum Studium des Reaktionsablaufes. Drug Res 19: 603–606
Heilbronn-Wikström E (1965) Phosphorylated cholinesterases. Svensk Kemisk Tidskr 77: 598–630
Hobbiger F (1963) Reactivation of phosphorylated acetylcholinesterase. In: Koelle GB (ed) Cholinesterases and anticholinesterase agents. Handbuch der experimentellen Pharmakologie Vol XV. Springer, Berlin Göttingen Heidelberg, pp 921–988
Hudson RF, Keay L (1960) The mechanism of hydrolysis of phosphonochloridates and related compounds. Part I. The effect of substituents. J Chem Soc: 1859–1865
De Jong LPA, Ceulen DI (1978) Anticholinesterase activity and rate of decomposition of some phosphylated oximes. Biochem Pharmacol 27: 857–863
Lamb JC, Steinberg GM, Hackley BE (1964) Isopropyl methylphosphonylated bisquaternary oximes: powerful inhibitors of cholinesterase. Biochim Biophys Acta 89: 174–176
Lamb JC, Steinberg GM, Solomon S, Hackley BE (1965) Reaction of 4-formyl-1-methylpyridinium iodide oxime with isopropyl methylphosphonofluoridate. Biochemistry 4: 2475–2484
Portmann R, Niederhauser A, Hofmann W, Frey A, Stoeckli-Evans H (1991) Synthesis of 4-[(isopropyloxy)methylphosphoryloxy]iminomethyl-1-methylpyridinium-iodide and its characterisation. Helv Chim Acta 74: 331–335
Scaife JF (1959) Oxime reactivation of inhibited true and pseudocholinesterase. Can J Biochem Physiol 37: 1301–1311
Schoene K (1976) Kinetic studies on chemical reactions between acetylcholinesterase, toxic organophosphates and pyridinium oximes. In: Medical protection against chemical warfare agents. SIPRI, Alqvist & Wiskell International, Stockholm, Sweden, pp 88–100
Tammelin LE (1957) Dialkoxy-phosphoryl-thiocholines, alkoxy-methyl-phosphoryl-thiocholines and analoguous choline esters. Acta Chem Scand 11: 1340–1349
Tammelin LE (1958) Organophosphorylcholines and cholinesterases. Arkiv för Kemi 12 [31]: 287–298
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Waser, P.G., Alioth-Streichenberg, C.M., Hopff, W.H. et al. Interaction of obidoxime with sarin in aqueous solution. Arch Toxicol 66, 211–215 (1992). https://doi.org/10.1007/BF01974017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01974017