Abstract
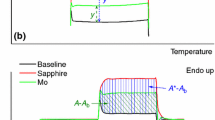
In DSC's the shape of the interpolated baseline under a peak is determined by a change in the heat capacity of the sample and the heat transfer characteristics between sample and temperature sensor. The interpolated baseline is constructed according to formal criteria, experimentally or analytically on the basis of physico-chemical assumptions on the change of the heat capacity during transition. By the example of the melting of ice this paper shows analytically on the basis of a simple calorimeter model and a synthetic measuring curve, and experimentally, that the uncertainty of the enthalpy determination depends in general on the type of baseline and is in the order of magnitude of the repeatability of the DSC's (±0.5%).
Zusammenfassung
In Differential-Scanning Kalorimetern wird die Gestalt der interpolierten Basislinie unter dem Peak durch eine Änderung der Wärmekapazität der Probe und die Wärmetransportcharakteristik zwischen Probe und Temperaturfühler bestimmt. Die interpolierte Basislinie wird nach formellen Kriterien konstruiert, experimentell oder analytisch auf der Grundlage von physikalisch-chemischen Annahmen für die Änderung der Wärmekapazität während des Überganges. Anhand des Beispieles schmelzendes Eis zeigt vorliegende Arbeit auf der Grundlage eines einfachen Kalorimetermodelles und einer synthetischen Meßkurve analytisch sowie experimentell, daß die Ungenauigkeit der Enthalpiebestimmung unter anderem von der Art der Basislinie abhängt und daß ihr Wert in die Größenordnung der Reproduzierbarkeit von Differential-Scanning Kalorimetern liegt (0.5%).
Similar content being viewed by others
References
W. F. Hemminger and H. K. Cammenga, ’Methoden der Thermischen Analyse’, Berlin, Springer 1989.
G. Adam and F. H. Müller, Kolloid Z. Z. Polym., 192 (1963) 29.
A. Engelter, Kolloid Z. Z. Polym., 205 (1965) 102.
J. P. Dumas, J. Phys. D.: Appl. Phys., 11 (1978) 1.
J. C. Van Miltenburg and M. A. Cuevas-Diarte, Thermochim. Acta 156 (1989) 291.
C. M. Guttman and J. H. Flynn, Anal. Chem., 45 (1973) 408.
W. P. Brennan, B. Miller and J. C. Whitwell, Ind. Eng. Chem. Fundam., 8 (1969) 314.
A. Doelman, A. R. Gregges and E. M. Barrall II, Analytical Calorimetry, Vol. 4, R. S. Porter, J. F. Johnson (Eds.), New York, Plenum Press 1977, p. 1–18.
J. Šesták, Wilson and Wilson's Comprehensive Analytical Chemistry, Vol. XII ’Thermal Analysis’, Part D, Amsterdam, Elsevier 1984, p. 310.
W. Hemminger and G. Höhne, Grundlagen der Kalorimetrie, Verlag Chemie, Weinheim, New York 1979.
G. B. Adams, Jr., H. L. Johnston and E. C. Kerr, J. Am. Chem. Soc., 74 (1952) 4784.
F. Gronvold, J. Thermal Anal., 13 (1978) 419.
F. Gronvold, Rev. Chim. Min., 11 (1974) 568.
F. Gronvold, Acta Chem. Scand, A 29 (1975) 945.
T. B. Douglas and J. L. Dever, J. Am. Chem. Soc., 76 (1954) 4824.
W. F. Giauque and J. W. Stout, J. Am. Chem. Soc, 58 (1936) 1144.
D. C. Ginnings and G. T. Furukawa, J. Am. Chem. Soc., 75 (1953) 522.
O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry, 5th ed. Oxford, Pergamon 1979.
G. W. H. Höhne, Thermochim. Acta, 69 (1983) 175.
S. Sarge, S. Bauerecker and H. K. Cammenga, Thermochim. Acta, 129 (1988) 309.
U. Bandara, J. Thermal Anal., 31 (1986) 1063.
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. H. J. Seifert on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Hemminger, W.F., Sarge, S.M. The baseline construction and its influence on the measurement of heat with differential scanning calorimeters. Journal of Thermal Analysis 37, 1455–1477 (1991). https://doi.org/10.1007/BF01913481
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01913481