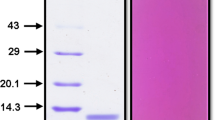
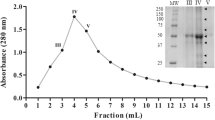
Abstract
Dipteryx alata trypsin inhibitor (DATI) has been purified and completely sequenced. It showed homology to members of the Bowman-Birk family of inhibitors. The last step of DATI purification by RP-HPLC (narrow-bore C18 column) suggested the existence of some isoforms of the inhibitor due to the presence of a cluster of very close peaks in the chromatogram. By using electrospray ionization mass spectrometry (ESIMS) and laser desorption mass spectrometry (LDIMS), the identification of DATI isoforms was made possible. From the ESIMS data, the following molecular masses were found: 6803.22±0.92 for isoforma; 6890.94±0.73 forb; 6977.58±0.39 for c; 7065.07±0.67 ford; 7151.42±0.86 for e; and 7291.70±0.43 forf. Similar masses were found when using LDIMS. Isoformb was the most abundant and its molecular mass matched the molecular mass of 6893 calculated from the sequence of DATI. The mass differences betweena andb, b andc, c andd, andd ande were equal to 87, which corresponds to Ser. Isoforma might not have the N-terminal Ser present in isoformb, while the other additional Ser residues might comprise a row localized in the C- or N-terminal. The appearance of all these isoforms could result from posttranslational N- and C-terminal processing.
Similar content being viewed by others
References
Aitken, A., Geisow, M. J., Findlay, J. B. C., Holmes, C., and Yarwood, A. (1989). InProtein Sequence—A Practical Approach (Findlay, J. B. C., and Geisow, M. J., eds.), Oxford University Press, Oxford, pp. 43–68.
Asao, T., Imai, F., Tsuji, I., Tashiro, M., Iwami, K., and Ibuki, F. (1991).J. Biochem. 110, 951–955.
Bode, W., and Huber, R. (1992).Eur. J. Biochem. 204, 433–451.
Bowles, D. J. (1990).Annu. Rev. Biochem. 59, 873–907.
Brown, W. E., Takio, K., Titani, K., and Ryan, C. A. (1985).Biochemistry 24, 2105–2108.
Chang, J. Y. (1983). InMethods in Enzymology, Vol. 91 (Hirs, C. H. W., and Timasheff, S. N., eds.), Academic Press, New York, pp. 455–466.
Chang, J. Y., Brauer, D., and Wittmann-Liebold, B. (1978).FEBS Lett. 93, 205–214.
Erlanger, B. F., Kokowsky, N., and Cohen, E. (1961).Arch. Biochem. Biophys. 95, 271–278.
Filgueiras, T. S., and Silva, E. (1975).Brasil Florestal 6, 33–39.
Garcia-Olmedo, F., Salcedo, G., Sanchez-Monge, R., Gomez, L., Royo, J., and Carbonero, P. (1987).Oxford Surv. Plant Mol. Cell Biol. 4, 275–334.
Hawke, D., and Yuan, P. (1987).Applied Biosystems User Bulletin, no. 28.
Higgins, D. G., and Sharp, P. M. (1989).Comput. Appl. Biosci. 5, 151–153.
Ikenaka, T., and Odani, S. (1978). InProceedings of the Symposium on Evolution of Protein Molecules (Matsubara, H., and Yamanaka, T., eds.), Japan Scientific Societies Press, Tokyo, pp. 287–296.
Irvine, G. B., and Shaw, C. (1986).Anal. Biochem. 155, 141–148.
Ishikawa, C., Nakamura, S., Watanabe, K., and Takahashi, K. (1979).FEBS Lett. 99, 97–100.
Jones, B. N. (1986). InMethods of Protein Microcharacterization—A Practical Handbook (Shively, J. E., ed.), Humana Press, Clifton, New Jersey, pp. 337–361.
Joubert, F. J. (1984).Phytochemistry 23, 957–961.
Joubert, F. J., Kruger, H., Townshend, G. S., and Botes, D. P. (1979).Eur. J. Biochem. 97, 85–91.
Kiyohara, T., Yokota, K., Masaki, Y., Matsui, O., Iwasaki, T., and Yoshikawa, M. (1981).J. Biochem. 90, 721–728.
Kollipara, K. P., and Hymowitz, T. (1992).J. Agric. Food Chem. 40, 2356–2363.
Kunitz, M. (1945/1946).J. Gen. Physiol. 29, 149–154.
Mandal, D. K., Nieves, E., Bhattacharyya, L., Orr, G. A., Rohoz, J., Yu, Q. T., and Brewer, C. F. (1994).Eur. J. Biochem. 221, 547–553.
Morhy, L. (1976). Inibidor Triptico e Quimotríptico deVigna sinensis L—Estudos por Espectroscopia de Diferença da Intera8c6ao com Beta-Tripsina e Alfa-Quimotripsina, M.Sc. „Interação com Beta-Tripsina e Alfa-Quimotripsina, M.Sc. thesis (abstract in English), Universidade de Braslilia, „thesis (abstract in English), Universidade de Brasília, Brasília.
Morhy, L., and Ventura, M. M. (1987).An. Acad. Bras. Ci. 59, 71–81.
Nielsen, S. S., and Liener, I. E. (1988).J. Food Sci. 53, 298–299.
Norioka, S., and Ikenaka, T. (1983).J. Biochem. 94, 589–599.
Odani, S., and Ikenaka, T. (1972).J. Biochem. 71, 839–848.
Odani, S., and Ikenaka, T. (1977).J. Biochem. 82, 1523–1531.
Odani, S., and Ikenaka, T. (1978).J. Biochem. 83, 737–745.
Odani, S., Koide, T., and Ono, T. (1986).J. Biochem. 100, 975–983.
Richardson, M. (1977).Phytochemistry 16, 159–169.
Richardson, M. (1991). InMethods in Plant Biochemistry, Vol. 5 (Rogers, L. J., ed.), Academic Press, New York, pp. 259–305.
Schägger, H., and von Jagow, G. (1987).Anal. Biochem. 166, 368–379.
Shimokawa, Y., Kuromizu, K., Araki, T., Ohata, J., and Abe, O. (1984).Eur. J. Biochem. 143, 677–684.
Stevens, F. C., Wuerz, S., and Krahn, J. (1974). InProteinase Inhibitors Bayer Symposium V (Fritz, H., Tschesche, H. T., Green, L. J., and Truscheit, E., eds.), Springer-Verlag, New York.
Tan, C. G. L., and Stevens, F. C. (1971).Eur. J. Biochem. 18, 503–514.
Tan-Wilson, A. L., Chen, J. C., Duggan, M. C., Chapman, C., Obach, R. S., and Wilson, K. A. (1987).J. Agric. Food Chem. 35, 974–981.
Thibault, P., Watson, D. C., Yaguchi, M., and Young, N. M. (1993). InTechniques in Protein Chemistry, Vol. IV (Angeletti, R. H., ed.), Academic Press, San Diego, pp. 91–98.
Weder, J. K. P. (1981). InAdvances in Legume Systematics, Part 2 (Polhill, R. M., and Raven, P. H., eds.), Royal Botanic Gardens, Kew, England, pp. 533–560.
Wilcox, M. D., Schey, K. L., Dingus, J., Mehta, N. D., Tatum, B. S., Halushka, M., Finch, J. W., and Hildebrandt, J. D. (1994).J. Biol. Chem. 269, 12508–12513.
Wilson, K. A., and Chen, J. C. (1983).Plant Physiol. 71, 341–349.
Wilson, K. A., and Laskowski, Jr. (1975).J. Biol. Chem. 250, 4261–4267.
Wilson, K. J., and Yuan, P. M. (1989). InProtein Sequence—A Practical Approach (Findlay, J. B. C., and Geisow, M. J., eds.), Oxford University Press, Oxford, pp. 1–41.
Wrigley, C. W. (1968).J. Chromatog. 36, 362–365.
Zhang, Y., Luo, S., Tan, E., Chi, C., Xu, L., and Zhang, A. (1982).Sci. Sin. (English translation)25, 268–276.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalume, D.E., Sousa, M.V. & Morhy, L. Purification, characterization, sequence determination, and mass spectrometric analysis of a trypsin inhibitor from seeds of the brazilian treeDipteryx alata (leguminosae). J Protein Chem 14, 685–693 (1995). https://doi.org/10.1007/BF01886907
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01886907