Abstract
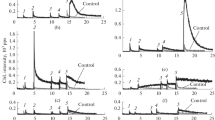
This investigation confirms the presence of the inducible mixed function hydroxylase enzyme system in nuclear membranes. The cytochrome P-450 spectrum and demethylase activity, markers of the enzyme system, were used to define its localization to the outer membrane envelope. Intact BALB/c mouse liver nuclei isolated and purified in Mg++ sucrose media of low ionic strength gave CO-dithionite reduced difference spectra of cytochrome P-450 and P-448. Phenobarbital induced P-450 by 40% while the carcinogenic hydrocarbon, benzo [α] pyrene, induced P-448 twofold. A corresponding increase was also observed in the microsomes of the same tissue preparations. No microsomal contamination of nuclear preparations was found. Intact nuclei stripped of their outer membrane by 0.5% Triton X-100 treatment resulted in a striking absence of the P-450 which, however, was found to be present in isolated outer nuclear membranes.
Similar content being viewed by others

References
C. Heidelberger and M. G. Moldenhauer,Cancer Res.,16 (1956) 442–449.
W. G. Wiest and C. Heidelberger,Cancer Res.,13 (1953) 255–261.
W. G. Wiest and C. Heidelberger,Cancer Res.,13 (1953) 250–254.
P. Brookes,Cancer. Res.,26 (1966) 1994–2003.
P. Brookes and P. D. Lawley,Nature (London)202 (1964) 781–784.
S. A. Lesko, Jr, O. P. Ts'o and R. S. Umans,Biochem.,8 (1969) 2291–2298.
H. V. Gelboin,Cancer Res.,29 (1969) 1272–1276.
H. Glaumann, B. Kuylenstierna and G. Dallner,Life Sci.,8 (1969) 1309–1315.
Y. Ichikawa and T. Yamano,Biochem. Biophys. Res. Commun.,40 (1970) 297–305.
L. D. Wilson, S. B. Oldham and B. W. Harding,J. Clin. Endocrinol. Metab.,28 (1968) 1143–1152.
M. K. Bach and H. G. Johnson,Biochem.,6 (1967) 1916–1933.
R. Berezney, L. K. Funk and F. L. Crane,Biochem. Biophys. Res. Commun.,38 (1970) 93–98.
R. Berezney, L. K. Funk and F. L. Crane,Biochim. Biophys. Acta.,203 (1970) 531–546.
W. W. Franke, B. Deumling, B. Ermen, E. Jarasch and H. Kleinig,J. Cell Biol.,46 (1970) 379–395.
C. B. Kasper,J. Biol. Chem.,246 (1971) 577–581.
A. S. Khandwala and C. B. Kasper,Biochem. Biophys. Res. Commun.,54 (1973) 1241–1246.
C. C. Windell and J. R. Tata,Biochem. J.,92, (1964) 313–317.
E. D. Whittle, D. E. Buchnell and V. R. Potter.Biochim. Biophys. Acta.,161 (1968) 41–50.
G. Blobel and V. R. Potter,Science,154 (1966) 1662–1665.
T. Omura and R. Sato inMethods in Enzymology (R. W. Estabrook, ed.)10 (1967) 556–561. Academic Press, New York.
A. G. Gornall, C. J. Bardawill and M. M. David,J. Biol. Chem.,177 (1949) 751–766.
C. W. Mehard, L. Packer and S. Abraham,Cancer Res.,31 (1971) 2148–2160.
D. M. Kashing and C. B. Kasper,J. Biol. Chem.,244 (1969) 3786–3792.
M. Bornens and C. B. Kasper,J. Biol. Chem.,248 (1973) 571–579.
H. Kleinig,J. Cell Biol.,46 (1970) 396–402.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehard, C.W., Packer, L. Induction of cytochrome P-450 and P-448 in the outer membrane of mouse liver nuclei by phenobarbital and benzo [α-]pyrene. J Bioenerg Biomembr 6, 151–160 (1974). https://doi.org/10.1007/BF01648982
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01648982