Summary
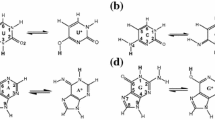
Computer-based models were derived for the covalent and noncovalent binding of the antitumor antibiotic quinocarcin to a representative DNA segment, d(ATGCAT)2. They showed that a mode of action, involving opening of the oxazolidine ring to give an iminium ion, followed by initial noncovalent binding in the minor groove and subsequent alkylation of the 2-amino group of guanine, was rational and attended by favorable interaction energies in each step. The best model had the aryl ring of quinocarcin lying in the 3′ direction from the covalent binding site and anR configuration at the carbon involved in covalent bond formation. It also showed that the preferred absolute configuration for quinocarcin was the reverse of that arbitrarily assigned in the literature.
Similar content being viewed by others
References
Tomita, F., Takahashi, K. and Shimizu, K., J. Antibiot., 35 (1983) 463–467.
Takahashi, K. and Tomita, F., J. Antibiot., 35 (1983) 468–470.
Hirayama, N. and Shirahata, K., J. Chem. Soc, Perkin Trans. 2(1983) 1705–1708.
Tomita, F., Takahashi, K. and Tamaoki, T., J. Antibiot., 37 (1984) 1268–1272.
Ishigoro, K., Takashashi, K., Yazawa, K., Sakiyama, S. and Arai, T., J. Biol. Chem., 256 (1981) 2162–2167.
Lown, I.W., Joshua, A.V. and Lee, J.S., Biochemistry 21 (1982) 419–428.
Zmijewski, Jr., M.J., Miller-Hatch, K. and Mikolajczak, M., Chem.-Biol. Interact., 52 (1985) 361–375.
Hurley, L.H., J. Antibiot., 30 (1977) 349–369.
Thurston, D.E. and Hurley, L.H., Drugs of the Future 8 (1983) 957–971.
Kishi, K., Yazawa, K., Takahashi, K., Mikami, Y. and Arai, T., J. antibiot., 37 (1984) 847–852.
Reynolds, V.L., Molineaux, I.J., Kaplan, D.J., Swenson, D.H. and Hurley, L.H., Biochemistry, 24 (1985) 6228–6237.
Weiner, P.K. and Kollman, P.A., J. Comput. Chem., 2 (1984) 287–303.
Weiner, S.J., Kollman, P.A., Case, D., Singh, U.C., Ghio, C., Alagona, G., Profeta Jr., S. and Weiner, P.K., J. Am. Chem. Soc, 106 (1984) 765–784.
Dewar, M.J.S. and Thiel, W., J. Am. Chem. Soc, 99 (1977) 4899–4907.
Clark, T., A Handbook of Computational Chemistry, Wiley, New York, NY, 1985.
Lown, J.W., Joshua, A.V. and Chen, H.-H., Can. J. Chem., 59 (1981) 2945–2952.
Langridge, R. and Ferrin, T.E., J. Mol. Graph, 2 (1984) 55–56.
Arnott, S., Campbell-Smith, P. and Chandrasekaron, R., In Fasman, G.D. (Ed.) CRC Handbook of Biochemistry, Vol. 2, CRC, Cleveland, OH, 1976 pp. 411–422.
Graves, D.E., Pattaroni, C., Krishnan, B.S., Ostrander, J.M., Hurley, L.H. and Krugh, T.R., J. Biol. Chem., 259 (1984) 8202–8209.
Cheatham, S., Kook, A., Hurley, L.H., Barkley, M. and Remers, W., J. Med. Chem., in press.
Remers, W.A., Mabilia, M. and Hopfinger, A.J., J. Med. Chem. 29 (1986) 2492–2503.
Evans, D.A., Illig, C.R. and Saddler, J.C., J. Am. Chem. Soc., 108 (1986) 2478–2479.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hill, G.C., Wunz, T.P. & Remers, W.A. Computer simulation of the binding of quinocarcin to DNA. Prediction of mode of action and absolute configuration. J Computer-Aided Mol Des 2, 91–106 (1988). https://doi.org/10.1007/BF01532085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01532085