Summary
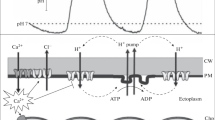
The potassium concentration was measured in the cytoplasm, perimicrovillar extracellular space (=‘vacuole’) and intercellular space of leech photoreceptors with double-barrelled potassium-sensitive microelectrodes in darkness and upon photostimulation. The mean intracellular potassium concentration in cells with membrane potentials >50 mV was 100±34 mmol/l. Photostimulation with 90 saturating 20 ms light flashes (1/s) evoked a potassium loss of 10.6±7.6 mmol/l. In the dark, there was no potassium concentration gradient between vacuole and intercellular space (K +VAC =4.5±0.9 mmol/l, K +ECS =4.5±0.5 mmol/l). In both compartments the potassium concentration increased upon repetitive photostimulation. Thus, the potassium loss from the cell is due to potassium movements across both the receptive and the non-receptive membrane domains.
The time courses of K+ accumulation and clearance differed in the two extracellular compartments: In the vacuole, potassium increased by 2.8±2.5 mmol/l to a ceiling level which was maintained during the standard train of light flashes. Potassium clearing in the dark was exponential with a half time of 60±26 s. In the intercellular space, repetitive photostimulation produced an initial rapid increase (half time <1 s) of the K+ concentration (mean† K +max =1.5±0.6 mmol/l). K+ clearing showed two superimposed components. A rapid one clears intercellular K+ after each light flash. The resultant K+ pulses ride on a slowly decreasing intercellular K+ level, and, following the last flash, K+ transiently undershoots the dark concentration.
Ouabain or a decrease in specimen temperature affect only the slow component and abolish the poststimulation K+ undershoot. Thus, the rapid component is interpreted as due to passive K+ dispersal by diffusion through the intercellular spaces, and the slow component and the poststimulation undershoot to K+ clearing by active reuptake of K+ into the photoreceptor cells.
K+ disappearance from the vacuole was not affected by ouabain, but a decrease in specimen temperature decreased the rate constant of K+ clearing, which has a Q10 of 1.48. It is concluded that K+ clearing from the vacuole is dominated by passive processes, and that the Na+/K+-pump is possibly localized only in the non-receptive membrane domain.
Similar content being viewed by others
References
Bracho H, Orkand RK (1972) Neuron-glia interaction: dependence on temperature. Brain Res 36:416–419
Brown JE, Lisman JE (1972) An electrogenic sodium pump inLimulus ventral photoreceptor cells. J Gen Physiol 59:720–733
Coles JA, Orkand RK (1982) Sodium activity in drone photoreceptors. J Physiol 332:16P
Coles JA, Orkand RK (1983) Modification of potassium movement through the retina of the drone (Apis mellifera) by glial uptake. J Physiol 340:157–174
Coles JA, Rick R (1985) An electron microprobe analysis of photoreceptors and outer pigment cells in the retina of the honeybee drone. J Comp Physiol A 156:213–222
Coles JA, Tsacopoulos M (1979) Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol 290:525–549
Coles JA, Tsacopoulos M (1981) Ionic and possible metabolic interactions between sensory neurons and glial cells in the retina of the honeybee drone. J Exp Biol 95:75–92
Coles JA, Tsacopoulos M, Rabineau P, Gardner-Medwin AR (1981) Movement of potassium into glial cells in the retina of the drone,Apis mellifera, during photostimulation. In: Syková E, Hink P, Vyklicky L (eds) Ion-selective microelectrodes, and their use in excitable tissues. Plenum Press, New York, pp 345–349
Deitmer JW, Schlue WR (1981a) Measurements of the intracellular potassium activity of Retzius cells in the leech central nervous system. J Exp Biol 91:87–101
Deitmer JW, Schlue WR (1981b) Active regulation of intracellular potassium in sensory neurons of the leech central nervous system. Naturwissenschaften 68:622
Deitmer JW, Schlue WR (1981c) Distribution of intra- and extracellular K+ in the leech central nervous system studied using double-barrelled ion-sensitive microelectrodes. In: Lübbers DW, Acker H, Buck RP, Eisenmann G, Kessler M, Simon W (eds) Progress in enzyme and ion-selective electrodes. Springer, Berlin Heidelberg New York, pp 93–99
Fain GL, Lisman JE (1981) Membrane conductances of photoreceptors. Prog Biophys Molec Biol 37:91–147
Fioravanti R, Fuortes MGF (1972) Analysis of responses in visuel cells of the leech. J Physiol 227:172–194
Galvan M, Dörge A, Beck F, Rick R (1984) Intracellular electrolyte concentrations in rat sympathetic neurons measured with an electron microprobe. Pflügers Arch 400:274–279
Hodgkin AL, Keynes RD (1955) The potassium permeability of a giant nerve fibre. J Physiol 128:61–88
Holt CE, Brown JE (1972) Ion fluxes in photoreception inLimulus polyphemus ventral eye. Biochim Biophys Acta 274:140–157
Lasansky A, Fuortes MGF (1969) The site of origin of electrical responses in visual cells of the leech,Hirudo medicinalis. J Cell Biol 42:241–252
Lewis DV, Schuette WH (1975) Temperature dependence of potassium clearance in the central nervous system. Brain Res 99:175–178
Munoz JL, Deyhimi F, Coles JA (1983) Silanization of glass in the making of ion-sensitive micro-electrodes. J Neurosci Meth 8:231–247
Nicholson C (1980) Dynamics of the brain cell microenvironment. Neurosci Res Prog Bull 18:177–322
Nicholson C, Bruggencate G ten, Senekowitsch R (1976) Large potassium signals and slow potentials evoked during aminopyridine or barium superfusion in cat cerebellum. Brain Res 113:606–610
Oehme M, Simon W (1976) Microelectrode for potassium ions based on a neutral carrier and comparison of its characteristics with a cation exchanger resin. Analyt Chim Acta 86:21–25
Stein WD (1967) The movement of molecules across the cell membranes. Academic Press, London New York San Francisco
Stieve H, Hartung K (1977) Kinetics of42K and86Rb loss from the crayfish retina in the dark and the effect of light on the rate of isotope loss. Biochim Biophys Acta 465:634–649
Syková E (1983) Extracellular K+ accumulation in the central nervous system. Prog Biophys Molec Biol 42:135–189
Taylor PS, Thomas RC (1984) The effect of leakage on microelectrode measurements of intracellular sodium activity in crab muscle fibres. J Physiol 352:539–550
Thomas RC (1978) Ion-sensitive intracellular microelectrodes. Academic Press, London New York San Francisco
Tsacopoulos M, Orkand RK, Coles JA, Levy S, Poitry S (1983) Oxygen uptake occurs faster than sodium pumping in bee retina after a light flash. Nature 301:604–606
Walz B (1984) K+ concentration in the cytoplasm, perimicrovillar extracellular space and intercellular space in leech photoreceptors in darkness and upon photostimulation. Verh Dtsch Zool Ges 77:342
Walz B, Somlyo AP (1984) Quantitative electron probe microanalysis of leech photoreceptors. J Comp Physiol A 154:81–87
White RH, Walther JB (1969) The leech photoreceptor cell: Ultrastructure of clefts connecting the phaosome with extracellular space demonstrated by lanthanum deposition. Z Zellforsch 95:560–562
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walz, B. Light-induced changes of extra- and intracellular potassium concentration in photoreceptors of the leech,Hirudo medicinalis . J. Comp. Physiol. 157, 199–210 (1985). https://doi.org/10.1007/BF01350027
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01350027