Summary
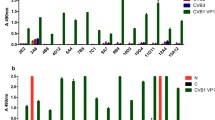
A type-specific monoclonal antibody was produced by immunizing mice with purified equid herpesvirus-1 (EHV-1). The EHV-1 specific mAb reacted with all the EHV-1 strains tested so far by indirect ELISA, immunofluorescence, and immunoperoxidase tests. No reactions were detected with the EHV-4, EHV-2, or EHV-3 strains tested. The indirect immunofluorescence and immunoperoxidase tests showed that the nuclei of infected cells were predominantly stained by this mAb. Triton treatment of the virus and immunogold labeling experiments indicated that the nucleocapsid of EHV-1 was the target antigen of the mAb. Preliminary results indicated that this mAb might be a useful tool in detecting specific antibody in horses that have been exposed to EHV-1. In a blocking ELISA, antibodies in sera from hamsters, mice and a foal which had been exposed to EHV-1 were differentiated from those in sera of animals exposed to EHV-4.
Similar content being viewed by others
References
Allen GP, Coogle LD, Ostlund EN, Yeargan MR (1992) Molecular dissection of two major equid herpesvirus-1 glycoprotein antigens (gB and gC) that elicit humoral immune responses in the horse. Proceedings of the Sixth International Conference on Equine Infectious Diseases, Cambridge 1991. R & W Publications, Newmarket, pp 181–195
Bonass WA, Elton DM, Halliburton IW, Killington RA, Meredith DM, Stocks JM, Taylor LA, Whittaker G (1992) Characterization of the glycoproteins of EHV-1. Proceedings of the Sixth International Conference on Equine Infectious Diseases. Cambridge 1991. R & W Publications, Newmarket, pp 283–291
Bowen B, Steinberg J, Laemmli UK, Weintraub H (1980) The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res 8: 1–20
Campbell TM, Studdert MJ (1983) Equine herpesvirus type 1. Vet Bull 53: 135–147
Crabb BS, Allen GP, Studdert MJ (1991) Characterization of the major glycoproteins of equine herpesviruses 4 and 1 and asinine herpesvirus-3 using monoclonal antibodies. J Gen Virol 72: 2075–2082
Edington N, Bridges CG, Broad SC (1987) Rapid diagnosis and characterization of equine herpesvirus-1 using monoclonal antibodies. Tierarztl Prax [Suppl] 2: 37–40
Gibson W, Roizman B (1972) Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol 10: 1044–1052
Guo P, Goebel S, Davis S, Perkus ME, Languet B, Desmettre P, Allen G, Paoletti E (1989) Expression in recombinant vaccinia virus of the equine herpesvirus-1 gene encoding glycoprotein gp 13 and protection of immunized animals. J Virol 63: 4189–4198
Jolly PD (1983) The development of an enzyme linked immunosorbent assay for the detection of antibodies to equid herpesvirus type 1 in horse sera. Bachelor of Philosophy Thesis, Massey University, New Zealand, pp 44–45
Kemp MC, Perdue ML, Rogers HW, O'Callaghan DJ, Randall CC (1974) Structural polypeptides of the hamster strain of equine herpesvirus type 1: products associated with purification. Virology 61: 361–375
Kit M, Kit S (1991) Sensitive glycoprotein g III blocking ELISA to distinguish between pseudorabies (Aujeszky's disease)-infected and vaccinated pigs. Vet Microbiol 28: 141–155
McLauchlan J, Rixon FJ (1992) Characterization of enveloped tegument structures (L-particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J Gen Virol 70: 1161–1172
Newcomb WW, Brown JC, Booy FP, Steven AC (1989) Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus-1: scanning transmission electron microscopic study. J Virol 63: 3777–3783
Nii S, Morgan C, Rose HM, Hsu KC (1968) Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol 2: 1172–1184
O'Keefe JS, Murray A, Wilks CR, Moriarty KM (1991) Amplification and differentiation of the DNA of an abortigenic (type 1) and a respiratory (type 4) strain of equine herpesvirus by the polymerase chain reaction. Res Vet Sci 50: 349–351
Ostlund EN, Allen GP, Yeargan MR, Coogle LD (1992) The antibody response of horses to specific antigenic domains on equid herpesvirus-1 glycoproteins B and C. Proceedings of the Sixth International Conference on Equine Infectious Diseases, Cambridge 1991. R & W Publications, Newmarket, pp 277–283
Perdue ML, Kemp MC, Randall CC, O'Callaghan DJ (1974) Studies of the molecular anatomy of the L-M cell strain of equine herpesvirus type 1: proteins of the nucleocapsid and intact virion. Virology 59: 201–216
Randall CC, Rogers HW, Downer DN, Gentry GA (1972) Protein kinase activity in equine herpesvirus. J Virol 9: 216–222
Sabine M, Robertson GR, Whalley JM (1981) Differentiation of the subtypes of equine herpesvirus-1 by restriction endonuclease analysis. Equine Vet J 57: 148–149
Sanchez-Martinez D, Schmid SD, Whittington W, Brown D, Reeves WC, Chatterjee S, Whitley RJ, Pellet PE (1991) Evaluation of a test based on baculovirus-expressed glycoprotein G for detection of herpes simplex virus type-specific antibodies. J Infect Dis 164: 1196–1199
Schloer GM, Breese SS (1982) Purification of African malignant catarrhal fever virus using a two phase aqueous polymer system. J Gen Virol 59: 101–110
Showalter SD, Zweig M, Hampar B (1981) Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate early protein ICP-4. Infect Immun 34: 684–692
Stokes A, Allen GP, Pullen LA, Murray PK (1989) A hamster model of equine herpesvirus type 1 (EHV-1) infection; passive protection by monoclonal antibodies to EHV-1 glycoproteins 13, 14, and 17/18. J Gen Virol 70: 1173–1183
Stokes A, Corteyn AH, Pullen LA, Doel TR, Meredith DM, Killington RA, Halliburton IW, Whittaker GR, Wheldon LA, Nicolson L, Onions DE, Allen GP, Murray PK (1991) Studies on glycoprotein 13 (gp 13) of equid herpesvirus-1 using affinity purified gp 13, glycoprotein-specific monoclonal antibodies and synthetic peptides in a hamster model. J Gen Virol 72: 923–931
Studdert MJ, Simpson T, Roizman B (1981) Differentiation of respiratory and abortigenic isolates of equine herpesvirus-1 by restriction endonucleases. Science 214: 256–264
Watson DH, Wildy P (1963) Some serological properties of herpes virus particles studied with the electron microscope. Virology 21: 100–111
Yao F, Courtney RJ (1989) A major transcriptional regulatory protein (ICP-4) of herpes simplex virus type 1 is associated with purified virions. J Virol 63: 3338–3344
Yeargan MR, Allen GP, Bryans JT (1985) Rapid subtyping of equine herpesvirus-1 with monoclonal antibodies. J Clin Microbiol 21: 694–697
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van de Moer, A., Rice, M. & Wilks, C.R. A type-specific conformational epitope on the nucleocapsid of equid herpesvirus-1 and its use in diagnosis. Archives of Virology 132, 133–144 (1993). https://doi.org/10.1007/BF01309848
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309848