Abstract
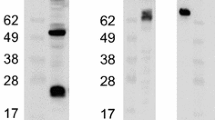
The ORF 70 gene of equid alphaherpesvirus type 3 (EHV-3) encodes glycoprotein G (gG), which is conserved in the majority of alphaherpesviruses. This glycoprotein is located in the viral envelope and has the characteristic of being secreted into the culture medium after proteolytic processing. It modulates the antiviral immune response of the host by interacting with chemokines. The aim of this study was to identify and characterize EHV-3 gG. By constructing viruses with HA-tagged gG, it was possible to detect gG in lysates of infected cells, their supernatants, and purified virions. A 100-, 60-, and 17-kDa form of the protein were detected in viral particles, while a 60-kDa form was identified in supernatants of infected cells. The role of EHV-3 gG in the viral infection cycle was assessed by the construction of a gG-minus EHV-3 mutant and its gG-positive revertant. When growth characteristics in an equine dermal fibroblast cell line were compared, the plaque size and the growth kinetics of the gG-minus mutant were similar to those of the revertant virus, suggesting that EHV-3 gG does not play a role in direct cell-to-cell transmission or virus proliferation of EHV-3 in tissue culture. The identification and characterization of EHV-3 gG described here provide a solid background for further studies to assess whether this glycoprotein has a function in modulating the host immune response.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Vissani MA, Damiani AM, Barrandeguy ME (2021) Equine coital exanthema: new insights on the knowledge and leading perspectives for treatment and prevention. Pathogens 10:1055. https://doi.org/10.3390/pathogens10081055
Davison AJ (2010) Herpesvirus systematics. Vet Microbiol 143:52–69. https://doi.org/10.1016/j.vetmic.2010.02.014
Sijmons S, Vissani A, Tordoya MS, Muylkens B, Thiry E, Maes P, Matthijnssens J, Barrandeguy M, Van Ranst M (2014) Complete genome sequence of equid herpesvirus 3. Genome Announc 2:e00797-14. https://doi.org/10.1128/genomeA.00797-14
Van de Walle GR, Jarosinski KW, Osterrieder N (2008) Alphaherpesviruses and chemokines: pas de deux not yet brought to perfection. J Virol 82:6090–6097. https://doi.org/10.1128/JVI.00098-08
Oliver SL, Yang E, Arvin AM (2016) Varicella-zoster virus glycoproteins: entry, replication, and pathogenesis. Curr Clin Microbiol Rep 3:204–215. https://doi.org/10.1007/s40588-016-0044-4
Viejo-Borbolla A, Martinez-Martín N, Nel HJ, Rueda P, Martín R, Blanco S, Arenzana-Seisdedos F, Thelen M, Fallon PG, Alcamí A (2012) Enhancement of chemokine function as an immunomodulatory strategy employed by human herpesviruses. PLoS Pathog 8:e1002497. https://doi.org/10.1371/journal.ppat.1002497
Bryant NA, Davis-Poynter N, Vanderplasschen A, Alcami A (2003) Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J 22:833–846. https://doi.org/10.1093/emboj/cdg092
Viejo-Borbolla A, Muñoz A, Tabarés E, Alcamí A (2010) Glycoprotein G from pseudorabies virus binds to chemokines with high affinity and inhibits their function. J Gen Virol 9:23–31. https://doi.org/10.1099/vir.0.011940-0
Akhmedzhanov M, Scrochi M, Barrandeguy M, Vissani A, Osterrieder N, Damiani AM (2017) Construction and manipulation of a full-length infectious bacterial artificial chromosome clone of equine herpesvirus type 3 (EHV-3). Virus Res 228:30–38. https://doi.org/10.1016/j.virusres.2016.11.012
Tischer BK, von Einem J, Kaufer B, Osterrieder N (2006) Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197. https://doi.org/10.2144/000112096
Hartley CA, Drummer HE, Studdert MJ (1999) The nucleotide sequence of the glycoprotein G homologue of equine herpesvirus 3 (EHV3) indicates EHV3 is a distinct equid alphaherpesvirus. Arch Virol 144:2023–2033. https://doi.org/10.1007/s007050050723
Barrandeguy M (2010) Virological aspects and pathogenesis of natural and experimental equid herpesvirus 3 infection in horses. Thesis D/20 ISBN 978–2-930404–79-0. Presses de la Faculté de Médecine vétérinaire de l’Université de Liège
Kirisawa R, Toishi Y, Akamatsu A, Soejima K, Miyashita T, Tsunoda N (2017) Isolation of equine herpesvirus 3 (EHV-3) from equine coital exanthema of two stallions and sero-epidemiology of EHV-3 infection in Japan. J Vet Med Sci 79:636–643. https://doi.org/10.1292/jvms.16-0518
Said A, Damiani A, Osterrieder N (2014) Ubiquitination and degradation of the ORF34 gene product of equine herpesvirus type 1 (EHV-1) at late times of infection. Virology 460–461:11–22. https://doi.org/10.1016/j.virol.2014.05.009
Said A, Osterrieder N (2014) Equine herpesvirus type 1 (EHV-1) open reading frame 59 encodes an early protein that is localized to the cytosol and required for efficient virus growth. Virology 20(449):263–269. https://doi.org/10.1016/j.virol.2013.11.035
Drummer HE, Studdert MJ, Crabb BS (1998) Equine herpesvirus-4 glycoprotein G is secreted as a disulphide-linked homodimer and is present as two homodimeric species in the virion. J Gen Virol 79:1205–1213. https://doi.org/10.1099/0022-1317-79-5-1205
Costes B, Thirion M, Dewals B, Mast J, Ackermann M, Markine-Goriaynoff N, Gillet L, Vanderplasschen A (2006) Felid herpesvirus 1 glycoprotein G is a structural protein that mediates the binding of chemokines on the viral envelope. Microbes Infect 8:2657–2667. https://doi.org/10.1016/j.micinf.2006.07.014
von Einem J, Smith PM, Van de Walle GR, O’Callaghan DJ, Osterrieder N (2007) In vitro and in vivo characterization of equine herpesvirus type 1 (EHV-1) mutants devoid of the viral chemokine-binding glycoprotein G (gG). Virology 362:151–162. https://doi.org/10.1016/j.virol.2006.12.008
Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T (1994) An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol 75:1245–1258. https://doi.org/10.1099/0022-1317-75-6-1245
Trapp S, Osterrieder N, Keil GM, Beer M (2003) Mutagenesis of a bovine herpesvirus type 1 genome cloned as an infectious bacterial artificial chromosome: analysis of glycoprotein E and G double deletion mutants. J Gen Virol 84:301–306. https://doi.org/10.1099/vir.0.18682-0
Azab W, El-Sheikh A, Abdel-Gawad A (2012) In vitro characterization of EHV-4 gG-deleted mutant. Virus Genes 44:109–111. https://doi.org/10.1007/s11262-011-0677-6
Demmin GL, Clase AC, Randall JA, Enquist LW, Banfield BW (2001) Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J Virol 75:10856–10869. https://doi.org/10.1128/JVI.75.22.10856-10869.2001
Acknowledgements
The authors thank the National Research Council (Conicet) and the University of Cuyo (UNCuyo) of Argentina for general support.
Funding
Financial support obtained from the Alberto J. Roemmers Foundation of Argentina made possible the completion of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, experiments, and analysis were performed by Antonella Losinno, Diego Sanchez, Maria Aldana Vissani, and Armando Mario Damiani. The first draft of the manuscript was written by Armando Mario Damiani, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals.
Additional information
Handling Editor: Akbar Dastjerdi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
705_2023_5727_MOESM1_ESM.tif
Supplementary Fig. S1 Detection of EHV-3 gG products using a v_tagN-infected cell lysate (lane 1) and purified viral proteins from v_tagN (lanes 2 and 3) and v_tagC (lane 4). The upper panel shows detection of gG, and the lower panel shows β-actin detection in the v_tagN-infected cell lysate to rule out cell debris contamination in the v_tagN and v_tagC purified viral protein samples. (TIF 287 KB)
705_2023_5727_MOESM2_ESM.tif
Supplementary Fig. S2 Detection of 100- and 60-kDa forms of the EHV-3 gG protein in purified viral protein preparations (lanes 2, 3, and 4) and supernatants (lanes 5, 6, and 7) of infected cells. The upper panel shows detection of gG, and the lower panel shows β-actin detection in v_tagN-infected cell lysates (lane 1) to rule out cell debris contamination in v_tagN and v_tagC purified viral proteins and supernatant samples. (TIF 258 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Losinno, A., Vissani, M.A., Sanchez, D. et al. Equid herpesvirus type 3 infection produces membrane-associated and secreted forms of glycoprotein G that are not required for efficient cell-to-cell spread of the virus in vitro. Arch Virol 168, 122 (2023). https://doi.org/10.1007/s00705-023-05727-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05727-4