Abstract
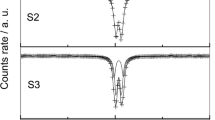
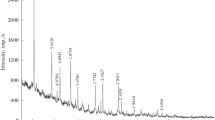
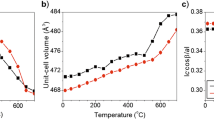
The structural properties of the solid phase, formed by the hydrolysis of Fe3+ ions in Fe2(SO4)3 solutions at 90 or 120 °C, were investigated using X-ray diffraction,57Fe Mössbauer spectroscopy, Fourier transform-infrared spectroscopy (FT—IR) and transmission electron microscopy. The concentration regions of Fe2(SO4)3 were determined for the precipitation of goethite, α-FeOOH, or hydronium jarosite, H3OFe3(OH)6(SO4)2′ as a single phase. Superparamagnetic behaviour of α-FeOOH particles was observed. Hydrolysis of Fe3+ ions in 0.1 M Fe2(SO4)3 solutions at 120 °C produced H3OFe3(OH)6(SO4)2 and basic sulphate, Fe4(OH)10SO4. The interpretation of57Fe Mössbauer and FT—IR spectra is given.
Similar content being viewed by others
References
H. Leidheiser, Jr andS. Musić,Corros. Sci. 22 (1982) 1089.
S. Musić, I. Czakó-Nagy, S. Popović, A. Vértes andM. Tonković,Croat. Chem. Acta 59 (1986) 833.
S. Musić, S. Popović andM. Gotić,ibid. 60 (1987) 661.
Idem, J. Mater. Sci. 25 (1990) 3186.
J. Dousma, D. Den Ottelander andP. L. De Bruyn,J. Inorg. Nucl. Chem. 41 (1979) 1565.
E. Matijević, R. S. Sapieszko andJ. B. Melville,J. Coll. Interface Sci. 50 (1975) 567.
R. S. Sapieszko, R. C. Patel andE. Matijević,J. Phys. Chem. 81 (1977) 1061.
S. Musić, A. Vértes, G. W. Simmons, I. Czakó-Nagy andH. Leidheiser Jr,J. Coll. Interface Sci. 85 (1982) 256.
N. Lazaroff, W. Sigal andA. Wassermann,Appl. Environ. Microbiol. 43 (1982) 924.
J. M. Bigham, U. Schwertmann, L. Carlson andE. Murad,Geochim. Cosmochim. Acta 54 (1990) 2743.
J. E. Dutrizac andS. Kaiman,Hydrometall. 1 (1975) 51.
J. E. Dutrizac andT. T. Chen,Canad. Mineral. 19 (1981) 559.
J. E. Dutrizac, in “Proceedings of the Australian Institute of Mining and Metallurgy, No. 278 (Australian Institute of Mining and Metallurgy, Parkville, Victoria 3052, Australia, 1981) pp. 23–32.
J. E. Dutrizac,Metall. Trans. B 14 (1983) 531.
J. E. Dutrizac, in “Hydrometallurgical Process Fundamentals” edited by R. G. Bautista (Plenum, 1984) pp. 125–69.
J. A. Ripmeester, C. I. Ratcliffe, J. E. Dutrizac andJ. L. Jambor,Canad. Mineral. 24 (1986) 435.
J. E. Dutrizac andJ. L. Jambor, in “Applied Mineralogy”, edited by W. C. Park, D. M. Hausen and R. D. Hagni (Metallurgical Society American Institute of Mining, Metallurgy and Petroleum Engineering, Warrendale, PA, 1985) pp. 507–30.
Idem, Hydrometall. 17 (1987) 251.
W. Kunda andH. Veltman,Metall. Trans B. 10 (1979) 439.
J. E. Dutrizac, in “Productivity and Technology in the Metallurgical Industries” edited by M. Koch and J. C. Taylor (Minerals, Metals and Materials Society, 1989) pp. 587–612.
S. Musić, A. Vértes, G. W. Simmons, I. Czakó-Nagy andH. Leidheiser Jr,Radiochem. Radioanal. Lett. 49 (1981) 315.
International Centre for Diffraction Data, Joint Committee on Powder Diffraction Standards, Powder Diffraction File, 1601 Park Lane, Swarthmore, PA 19081, USA.
A. Leclerc,Phys. Chem. Mineral. 6 (1980) 327.
J. R. Gancedo andM. L. Martinez, in “Magnetic Resonance in Colloid and Interface Science” edited by J. P. Fraissard and H. A. Resing (Reidel, 1980) p. 371.
C. Morterra, A. Chiorino andE. Borello,Mater. Chem. Phys. 10 (1984) 119.
L. Verdonck, S. Hoste, F. F. Roelandt andG. P. Van Der Kelen,J. Molec. Struct. 79 (1982) 273.
P. Cambier,Clay Minerals 21 (1986) 191.
S. Musić, J. Šipalo-Žuljević andR. H. H. Wolf,Radiochim. Radioanal. Lett. 45 (1980) 235.
S. Musić, J. Šipalo-Žuljević,Radiochim. Acta 27 (1980) 61.
S. Musić, M. Ristić andM. Tonković,Z. Waser-Abwaser Forsch. 19 (1986) 186.
C. J. Serna, C. Parada Cortina andJ. V. Garcia-Ramos,Spectrochim. Acta 42A (1986) 729.
I. L. Botto, E. J. Baran andA. C. Garcia,An. Quim. 83B (1987) 145.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Musić, S., Orehovec, Z., Popović, S. et al. Structural properties of precipitates formed by hydrolysis of Fe3+ ions in Fe2(SO4)3 solutions. J Mater Sci 29, 1991–1998 (1994). https://doi.org/10.1007/BF01154672
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01154672