Abstract
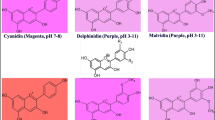
Precursor experiments and chromatographic studies indicate that the hydroxylation of flavanones in the 3-position to dihydroflavonols is blocked in special white-flowering mutants ofDahlia, Streptocarpus, Verbena andZinnia. The result of our investigations was confirmed in as much as the activity of the enzyme flavanone 3-hydroxylase, which catalyses the conversion of flavanones to dihydroflavonols, could readily be detected in flower extracts of cyanic strains of the four plant species. It was found to be, however, completely absent in flower extracts of the corresponding acyanic mutants. Thus, the interruption of the anthocyanin pathway in these mutants is clearly caused by a lack of this enzyme activity. Similar to the enzymes from other sources, the 3-hydroxylases ofDahlia, Streptocarpus, Verbena andZinnia are soluble enzymes; they belong to the 2-oxoglutarate-dependent dioxygenases and the reaction is inhibited by ethylenediaminetetraacetic acid, KCN and diethyldithiocarbamate.
Similar content being viewed by others
References
Abbott, M.T., Udenfriend, S. (1974) α-ketoglutarate-coupled dioxygenases. In: Molecular mechanisms of oxygen activation, pp. 167–214, Hayaishi, O., ed. Academic Press, New York London
Barz, W., Wiermann, R. (1981) Recent aspects of flavonoid biosynthesis and degradation in plants. In: Proceedings of the International Bioflavonoid Symposium, pp. 185–211, Munich 1981
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254
Britsch, L., Heller, W., Grisebach, H. (1981) Conversion of flavanone to flavone, dihydroflavonol and flavonol with an enzyme system from cell cultures of parsley. Z. Naturforsch. Teil C36, 742–750
Ebel, J., Hahlbrock, K. (1982) Biosynthesis. In: The flavonoids, advances in research, pp. 641–679, Harborne, J.B., Mabry, T.J., eds. Chapman & Hall, London New York
Forkmann, G. (1977) Precursors and genetic control of anthocyanin synthesis inMatthiola incana R. Br. Planta137, 159–163
Forkmann, G., Heller, W., Grisebach, H. (1980) Anthocyanin biosynthesis in flowers ofMatthiola incana. Flavanone 3-and flavonoid 3′-hydroxylases. Z. Naturforsch. Teil C35, 691–695
Forkmann, G., Stotz, G. (1981) Genetic control of flavanone 3-hydroxylase activity and flavonoid 3′-hydroxylase activity inAntirrhinum majus (Snapdragon). Z. Naturforsch. Teil C36, 411–416
Harborne, J.B. (1967) Comparative biochemistry of the flavonoids. Academic Press, London New York
Mabry, T.B., Markham, K.R., Thomas, M.B. (1970) The systematic identification of flavonoids. Springer, Berlin Heidelberg New York
Seyffert, W. (1982) Beiträge zur Genetik und Enzymologie der Flavonoide. Biol. Zentralbl.101, 465–483
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Forkmann, G., Stotz, G. Selection and characterisation of flavanone 3-hydroxylase mutants ofDahlia, Streptocarpus, Verbena andZinnia . Planta 161, 261–265 (1984). https://doi.org/10.1007/BF00982923
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00982923