Abstract
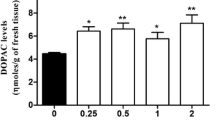
The effects on rat striatal dopamine (DA) metabolism of systemic and local administration of CGP 28014, an inhibitor of catechol-O-methyl-transferase (COMT), were studied by in vivo microdialysis. CGP 28014 (30 mg/kg i.p.) significantly reduced the levels of homovanillic acid (HVA), but did not modify DA and 3,4-dihydroxyphenylacetic acid (DOPAC). The intrastriatal administration (via the microdialysis probe) of 5, 7.5, 10, and 20 mM of CGP 28014 elicited a concentration-dependent, several-fold increase in extracellular DA but did not alter the levels of HVA and DOPAC. Thus, the effects of CGP 28014 observed after i.p. injection (decrease in HVA levels) are different from those measured after intrastriatal administration (increase in DA release). Therefore, the inhibition of COMT is likely to be due to the action of a metabolite of CGP 28014 formed in the periphery and not in the brain.
Similar content being viewed by others
References
Männistö, P. T., and Kaakkola, S. 1989. New selective COMT inhibitors: useful adjuncts for Parkinson's disease? TIPS 10:54–56.
Waldmeier, P. C., Baumann, P. A., Feldtrauer, J. J., Hauser, K., Bittiger, H., Bischoff, S., and von Sprecher, G. 1990. CGP 28014, a new inhibitor of cerebral catechol-O-methylation with a non-catechol structure. Naunyn-Schmiedeberg's Arch. Pharmacol. 342:305–311.
Guttman, M., Léger, G., Cedarbaum, J. M., Reches, A., Woodward, W., Evans, A., Diksi, M., and Gjedde, A. 1992. 3-O-methyldopa administration does not alter fluorodopa transport into the brain. Ann. Neurol. 31:638–643.
Zigmond, M. J., Hastings, T. G., and Abercrombie, E. D. 1992. Neurochemical response to 6-hydroxydopamine and L-dopa therapy: implications for Parkinson's disease. Ann. N.Y. Acad. Sci. 648:71–86.
Waldmeier, P. C., De Herdt, P., and Maitre, L. 1990. Effects of the COMT inhibitor, CGP 28014, on plasma homovanillic acid and O-methylation of exogenous L-DOPA in the rat. J. Neurol Transm. [Suppl] 32:381–386.
Ungerstedt, U. 1991. Microdialysis-principles and applications for studies in animals and man. J. Internal Medicin 230:365–373.
Westerink, B. H. C., Damsma, G., Rollema, H., De Vries, J. B., and Horn, A. S. 1987. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 41:1763–1776.
Pellegrino, L. J., Pellegrino, A. S., and Cushman, A. J. (eds.) 1979. Stereotaxic atlas of the rat brain, page 22, Plenum Press, New York.
Farnebo, L. O. 1971. Release of monoamines evoked by field stimulation. Studies on some ionic and metabolic requirements. Acta Physiol. Scand. [Suppl] 371:19–27.
Waldmeier, P. C., Williams, M., Baumann, P. A., Bischoff, S., Sills, M. A., and Neale, R. F. 1988. Interactions of isamoltane (CGP 361 A), an anxiolytic phenoxypropanolamine derivative, with 5-HT1 receptor subtypes in the rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 337:609–620.
Winer, B. J. 1971. Statistical Principles in Experiment Design. McGraw-Hill, New York.
Altman, P. L., and Dittmer, D. S. (eds.) 1974. Biology Data Book, Vol. 3, page 1847, Federation of American Societies for Experimental Biology, Bethesda.
Meek, J. L., and Neff, N. H. 1973. Is cerebrospinal fluid the major avenue for the removal of 5-hydroxyindoleacetic acid from the brain? Neuropharmacology 12:497–499.
Nomikos, G. G., Damsma, G., Wenkstern, D., and Fibiger, H. C. 1990. In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse 6:106–112.
Glowinski, J. 1970. Effects of amphetamine on various aspects of catecholamine metabolism in the central nervous system of the rat. Pages 301–306,in Costa, E., and Garattini, S., (eds.), Amphetamines and related compounds, Raven Press, New York.
Acquas, E., Carboni, E., de Ree, R. H. A., Da Prada, M., and Di Chiara, G. 1992. Extracellular concentrations of dopamine and metabolites in the rat caudate after oral administration of a novel catechol-O-methyltransferase inhibitor Ro 40-7592. J. Neurochem. 59:326–330.
Männistö, P. T., Tuomainen, P., and Tuomainen, R. K. 1992. Different in vivo properties of three new inhibitors of catechol O-methyltransferase in the rat. Br. J. Pharmacol. 105:569–574.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steulet, AF., Stöcklin, K., Wicki, P. et al. Effects of CGP 28014 on the in vivo release and metabolism of dopamine in the rat striatum assessed by brain microdialysis. Neurochem Res 18, 1131–1136 (1993). https://doi.org/10.1007/BF00978363
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00978363