Conclusions
-
1.
4-O-(p-Tolyldiphenylmethyl)-, 4-O-(di-p-tolylphenylmethyl) and 4-O-(p-anisyldiphenyl-raethyl) derivatives of methyl-2,3,-di-O-acetyl-β-D-xylopyranoside were synthesized.
-
2.
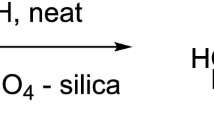
A study of the reaction of the above compounds with 3,4-di-O-acetyl-1,2-O-[1-(endo-cyano)ethylidene]-α-D-xylopyranose showed that exchange of the trityl group in methyl-2,3-di-O-acetyl-4-O-trityl-β-D-xylopyranoside for triarylmethyl groups containing electron-donor substituents in the aromatic ring does not appreciably affect the stereochemistry of the glycosylation, but can lead to decrease in the yield of the disaccharides.
-
3.
A trans-triarylmethylation reaction was discovered with the reaction of methyl-2,3-di-O-acetyl-4-O-(p-anisyldiphenylmethyl)-β-D-xylopyranoside with triphenylmethylium perchlorate as an example.
Similar content being viewed by others
Literature cited
N. K. Kochetkov, Izv. Akad. Nauk SSSR, Ser. Khim., 1543 (1982).
L. V. Bakinovskii, N. E. Nifant'ev, and N. K. Kochetkov, Bioorg. Khim.,9, 1089 (1983).
L. V. Bakinovskii, N. E. Nifant'ev, and K. Kochetkov, Bioorg. Khim.,10, 226 (1984).
L. V. Bakinovskii, N. E. Nifant'ev, V. I. Betaneli, M. I. Struchkova, and N. K. Kochet-kov, Bioorg. Khim.,9, 74 (1983).
J. K. N. Jones and M. Stacey, J. Chem. Soc., 1341 (1947).
P. V. Kent, M. Stacey, and L. F. Wiggins, J. Chem. Soc., 1232 (1949).
P. Kovac, J. Hirsch, and V. Kovacik, Chem. Zvesti,32, 514 (1978).
H. J. Douben, Jr., L. R. Honnen, and K. M. Harmon, J. Org. Chem.,25, 1442 (1960).
N. K. Kochetkov, V. I. Betaneli, M. V. Ovchinnikov, and L. V. Backinowsky, Tetrahedron,37, Suppl. 9, 149 (1981).
S. F. Acree, Chem. Ber.,37, 990 (1904).
G. V. Epple, V. P. Odintsova, and S. G. Entelis, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 1365 (1962).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 914–920, April, 1987.
Rights and permissions
About this article
Cite this article
Nifant'ev, N.E., Bakinovskii, L.V., Tsvetkov, Y.E. et al. Glycosylation of methyl-2,3-di-O-acetyl-β-D-xylopyranoside triarylmethyl ethers with electron-donor substituents in the aromatic ring by 3,4-di-O-acetyl-1,2-O-[1-(endo-cyano)-ethylidene]-α-D-xylo-pyranose. Russ Chem Bull 36, 840–847 (1987). https://doi.org/10.1007/BF00962335
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00962335