Abstract
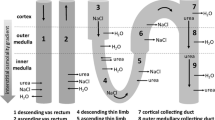
Under normal physiological conditions, demands placed on mammalian renal cortical cells are quite different from those in the medulla. Cortical proximal tubule cells exist in an isotonic environment, but must resorb vast amounts of filtered fluid and solute, and also adjust to solute generated from cellular metabolism. In addition, cortical cells must also adjust to occasional pathological derangements in blood osmolality. By contrast, human medullary cells have a smaller solute resorptive load, but exist in a milieu where osmolality varies from 40 to more than 1200 mosmol/kg H2O, depending on water intake. Remarkably, the cells maintain a near normal size despite these stresses. Under isosmotic conditions, the primary regulator of cell volume is Na-K ATPase. In its absence, factors such as external protein, extracellular matrix and basement membrane, cytoskeleton, and perhaps formation of cytoplasmic vesicular-like structures help prevent cells from swelling massively. Under anisosmotic conditions, a variety of transport processes operating across basolateral and apical membranes either remove solute from or add solute (and water) to cells to minimize changes in their size. Medullary cells have the additional ability to accumulate organic, non-toxic, osmolytes that offset external hypertonicity and allow cells to maintain normal size without increasing cellular inorganic ion concentrations.
Similar content being viewed by others
References
Macknight ADC (1987) Volume maintenance in isosmotic conditions. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 3–43
Macknight ADC (1988) Principles of cell volume regulation. Renal Physiol Biochem 3–5:114–141
Linshaw MA, Stapleton FB, Cuppage FE, Grantham JJ (1977) Effect of basement membrane and colloid osmotic pressure on renal tubule cell volume. Am J Physiol 233:F325-F332
Pine MB, Brooks WW, Nosta JJ, Abelmann WH (1981) Hydrostatic forces limit swelling of rat ventricular myocardium. Am J Physiol 241:H740-H747
Spyropoulos CS (1979) Cytoplasmic gel and water relations of axon. J Membr Biol 47:195–238
Geiger B (1983) Membrane-cytoskeletal interaction. Biochim Biophys Acta 737:305–341
Kleinzeller A (1972) Cellular transport of water. In: Hokin LE (ed) Metabolic pathways, 3rd edn, vol. VI, Metabolic transport. Academic Press, New York, pp 91–131
Leaf A (1959) Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci 72:396–404
Macknight ADC, Leaf A (1977) The regulation of cellular volume. Physiol Rev 57:510–573
Jacobson HR (1987) Ion transport in proximal nephron segments. In: Brenner B, Stein J (eds) Modern techniques of ion transport. Churchill Livingstone, New York, pp 199–235
Linshaw MA (1989) Volume control of isolated rabbit proximal tubules. Semin Nephrol 9:83–90
Dellasega M, Grantham JJ (1973) Regulation of renal tubule cell volume in hypotonic media. Am J Physiol 224:1288–1294
Linshaw MA (1980) Effect of metabolic inhibitors on renal tubule cell volume. Am J Physiol 239:F571-F577
Lohr JW, Sullivan LP, Cragoe EJ Jr, Grantham JJ (1989) Volume regulation determinants in isolated proximal tubules in hypertonic medium. Am J Physiol 256:F622-F631
Sullivan LP, Wallace DP, Clancy RL, Grantham JJ (1990) Effect of cellular acidosis on cell volume in S2 segments of renal proximal tubules. Am J Physiol 258:F831-F839
Cooke KR, Macknight ADC (1984) Effects of medium acetate on cellular volume in rabbit renal cortical slices. J Physiol (Lond) 349: 135–156
Macknight ADC (1985) The role of anions in cellular volume regulation. Pflugers Arch [Suppl] 405:S12-S16
Glynn IM, Karlish DJD (1975) The sodium pump. Annu Rev Physiol 37:13–55
Linshaw MA, Stapleton FB (1978) Effect of ouabain and colloid osmotic pressure in renal tubule cell volume. Am J Physiol 235: F480-F491
Linshaw MA, Welling LW (1983) Basolateral membrane properties in proximal convoluted tubules of the newborn rabbit. Am J Physiol 244:F172-F177
Linshaw MA, Bauman CA, Welling LW (1986) Use of trypan blue for identifying early proximal convoluted tubules. Am J Physiol 251:F214-F219
Linshaw MA, Welling LW, Bauman CA (1986) Basolateral membrane properties of juxtamedullary proximal tubule in newborn rabbit. Am J Physiol 251:F208-F213
Stapleton FB, Linshaw MA (1978) Regulation of cell volume in a single human proximal straight tubule. Renal Physiol 1:334–337
Cooke KR (1981) Ouabain and regulation of cellular volume in slices of mammalian renal cortex. J Physiol 320:319–332
Daniel EE, Robinson K (1971) Effects of inhibitors of active transport on22Na and42K movements and on nucleotide levels in rat uteri at 25° C. Can J Physiol Pharmacol 48:178–204
Hoffman JF, Kregenow FM (1966) The characterization of new energy-dependent cation transport processes in red blood cells. Ann N Y Acad Sci 137:566–576
Kleinzeller A, Knotkova A (1964) The effect of ouabain on the electrolyte and water transport in kidney cortex and liver slices. J Physiol (Lond) 175:172–192
Kleinzeller A, Knotkova A (1964) Electrolyte transport in rat diaphragm. Physiol Bohemoslov 13:317–326
Macknight ADC, Pilgrim JP, Robinson BA (1974) The regulation of cellular volume in liver slices. J Physiol (Lond) 238:279–294
Macknight ADC (1968) Water and electrolyte contents of rat renal cortical slices incubated in potassium-free media and media contatining ouabain. Biochim Biophys Acta 150:263–270
Macknight ADC (1983) Volume regulation in mammalian kidney cells. Mol Physiol 4:17–31
Whittam R, Willis JA (1963) Ion movements and oxygen consumption in kidney cortex slices. J Physiol (Lond) 168:158–177
Whittembury G (1965) Sodium extrusion and potassium uptake in guinea pig kidney cortex slices. J Gen Physiol 48:669–717
Paillard M, Leviel F, Gardin JP (1979) Regulation of cell volume in separated renal tubules incubated in hypotonic medium. Am J Physiol 236:F226-F231
Podevin RA, Boumendil-Podevin EF (1972) Effects of temperature, medium K+, ouabain and ethacrynic acid on transport of electrolytes and water in separated renal tubules. Biochim Biophys Acta 282:234–249
Rorive G, Nielsen R, Kleinzeller A (1972) Effect of pH on the water and electrolyte content of renal cells. Biochim Biophys Acta 266: 376–396
Mudge GH (1951) Electrolyte and water metabolism of rabbit kidney slices: effect of metabolic inhibitors. Am J Physiol 167: 206–223
Whittembury G (1968) Sodium and water transport in kidney proximal tubular cells. J Gen Physiol 51:3035–3143
Whittembury G, Proverbio F (1970) Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pfluegers Arch 316: 1–25
Marin R, Proverbio T, Proverbio F (1985) Active sodium transport in basolateral plasma membrane vesicles from rat kidney proximal tubular cells. Biochim Biophys Acta 814:363–373
Proverbio F, Duque JA, Proverbia T, Marin R (1988) Cell volume sensitive Na+-ATPase activity in rat kidney cortex membranes. Biochim Biophys Acta 941:107–110
Willis JS (1968) The interaction of K+, ouabain and Na+ on the cation transport and respiration of renal cortical slices of hamsters and ground squirrels. Biochim Biophys Acta 163:516–530
Mills JW, Macknight ADC, Jarrell JA, Dayer JM, Ausiello DA (1981) Interaction of ouabain with a Na+ pump in intact epithelial cells. J Cell Biol 88:637–643
Welling LW, Grantham JJ (1972) Physical properties of isolated perfused renal tubules and tubular basement membranes. J Clin Invest 51:1063–1075
Grantham JJ (1970) Vasopressin: effect on deformity of urinary surface of collecting duct cells. Science 168:1093–1095
Rand RP, Burton AC (1964) Mechanical properties of the red cell membrane. I. Membrane stiffness and intracellular pressure. Biophys J 4:115–135
Linshaw MA, Macalister TJ, Welling LW, Bauman CA, Hebert GZ, Downey GP, Koo EWY, Gotlieb AI (1991) Role of cytoskeleton in cell volume control of rabbit proximal tubules. Am J Physiol (in press)
Civan MM (1981) Intracellular potassium in toad urinary bladder: the recycling hypothesis. In: Macknight ADC, Leader JP (eds) Epithelial ion and water transport. Raven, New York, pp 107–116
Jorgensen PL (1980) Sodium and potassium ion pump in kidney tubules. Physiol Rev 60:864–917
Doucet A (1988) Function and control of Na−K ATPase in single nephron segments of the mammalian kidney. Kidney Int 34: 749–760
Kumar S, Berg JA, Katz AA (1991) Na:K pump abundance and function in MDCK cells: effect of low ambient potassium. Renal Physiol Biochem 14:19–27
Cardinal J, Duchesneau D (1978) Effect of potassium on proximal tubular function. Am J Physiol 234:F381-F385
Biagi B, Kubota T, Sohtell M, Giebisch G (1981) Intracellular potentials in rabbit proximal tubules perfused in vitro. Am J Physiol 240:F200-F210
Gagnon J, Ouimet D, Nguyen H, Laprade R, LeGrimellec C, Carriere S, Cardinal J (1982) Cell volume regulation in the proximal convoluted tubule. Am. J Physiol 243:F408-F415
Comper WD, Laurent TC (1978) Physiological function of connective tissue polysaccharides. Physiol Rev 58:255–315
Law RO (1984) Characteristics of ionic binding by rat renal tissue in vitro. J Physiol (Lond) 353:67–80
Law RO (1984) The influence of age on fluid and sodium chloride distribution in rat aortic wall. Q J Exp Physiol 69:737–751
Kanwar YS, Farquhar MG (1980) Detachment of endothelium and epithelium from the glomerular basement membrane produced by kidney perfusion with neuraminidase. Lab Invest 42:375–384
Stow JL, Sawada H, Farquhar MG (1985) Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae in podocytes of the rat renal glomerulus. Proc Natl Acad Sci USA 82:3296–3300
Reubsaet FAG, Langeveld JPM, Veerkamp JH (1985) Glycosaminoglycan content of glomerular and tubular basement membranes of various species. Biochim Biophys Acta 838:144–150
Bershadsky AD, Vasiliev JM (1988) In: Siekevitz P (ed) Cytoskeleton. Cellular organelles series, 2nd edn. Plenum, New York, pp 4, 133
Mills JW (1987) The cell cytoskeleton: possible role in volume control. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 75–101
Mills JW, Skiest DJ (1985) Role of cyclic AMP and the cytoskeleton in volume control in MDCK cells. Mol Physiol 8:247–262
Melmed RN, Karanian PJ, Berlin RD (1981) Control of cell volume in the J774 macrophage by microtubule disassembly and cyclic AMP. J Cell Biol 90:761–768
Rossum GDV van, Russo MA (1981) Ouabain-resistant mechanism of volume control and the ultrastructural organization of liver slices recovering from swelling in vitro. J Membr Biol 59:191–209
Foskett JK, Spring KR (1985) Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol 248:C27-C36
Gilles R, Delpire E, Duchene C, Cornet M, Pequex A (1986) The effect of cytochalasin B on the volume regulation response of isolated axons of the green crabCarcinus maenas submitted to hypoosmotic media. Comp Biochem Physiol [A] 85:523–525
Cornet M, Delpire E, Gilles R (1988) Relations between cell volume control and microfilaments networks in T2 and P12 cultured cells. J Physiol (Paris) 83:43–49
Kevers C, Pequex A, Gilles R (1979) Effects of an hypo-osmotic shock on Na+, K+ and CI-levels in isolated axons ofCarcinus maenas. J Comp Physiol 129:365–371
Cherksey BD, Zadunaisky JA (1981) Membrane beta-receptors: interaction with cytoskeleton in chloride secretory systems. In: Scott WN, Goodman DBP (eds) Hormonal regulation of epithelial transport of ions and water. Ann NY Acad Sci 372:309–331
Schliwa M (1986) The cytoskeleton. In: Alfert M, Beermann W, Goldstein L, Porter KR (eds) Cell biology monographs, vol. 13 Springer, New York
Rapraeger A, Jalkanen M, Bernfield M (1986) Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol 103: 2683–2696
Dustin P (1984) Microtubules. Springer, Berlin, pp 171–233
Hartwig JH, Stossel TP (1979) Cytochalasin B and the structure of actin gels. J Mol Biol 134:539–553
DeBrabander M, Geuens G, Nuydens R, Willebrords R, Moeremans M, van Ginckel R, Distelmans W, Dragonetti C, Mareel M (1986) Tubulozole: A new stereoselective microtubule inhibitor. Ann NY Acad Sci 466:757–766
Geuens GMA, Nuydens RM, Willebrords RE, Viere RM van de, Goosens F, Dragonetti CH, Mareel MM, DeBrabander MJ (1985) The effects of tubulozole on the microtubule system of cells in culture and in vivo. Cancer Res 45:733–742
Schliwa M (1982) Action of cytochalasin D on cytoskeletal networks. J Cell Biol 92:79–91
Rampal AL, Pinkofsky HB, Jung CY (1980) Structure of cytochalasins and cytochalasin B binding sites in human erythrocyte membranes. Biochemistry 19:679–683
Russo MA, Ernst SA, Kapoor SC, Rossum GDV van (1985) Morphological and physiological studies of rat kidney cortex slices undergoing isosmotic swelling and its reversal: a possible mechanism for ouabain-resistant control of cell volume. J Membr Biol 85: 1–24
Janoshazi A, Seifter JL, Solomon AK (1989) Interactions between anion exchange and other membrane proteins in rabbit kidney medullary collecting duct cells. J Membr Biol 112:39–49
Van Rossum GDV, Russo MA, Schisselbauer JC (1987) Role of cytoplasmic vesicles in volume maintenance. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 45–74
Okazaki Y, Tazawa M (1990) Calcium ion and turgor regulation in plant cells. J Membr Biol 114:189–194
Segawa A, Yamashina S (1989) Role of microfilaments in exocytosis: a new hypothesis. Cell Struct Funct 14:531–544
Lewis SA, Moura JLC de (1982) Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature 297:685–688
Gilles R, Duchene C, Lambert I (1983) Effect of ouabain on volume regulation of rabbit kidney cortex slices in hypo-osmotic media. Experientia 39:600–602
Seel M, Rorive G, Pequeux A, Gilles R (1980) Effect of a hypoosmotic shock on the volume and the ion content of rat kidney cortex slices. Comp Biochem Physiol [A] 65:29–33
Grantham JJ, Linshaw MA (1984) The effect of hyponatremia on the regulation of intracellular volume and solute composition. Circ Res 54:483–491
Linshaw MA, Grantham JJ (1980) Effect of collagenase and ouabain on renal cell volume in hypotonic media. Am J Physiol 238:F491-F498
Lohr JW, Grantham JJ (1986) Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J Clin Invest 78: 1165–1172
Grinstein S, Rothstein A, Sarkadi B, Gelfand EW (1984) Responses of lymphocytes to anisotonic media: volume regulating behavior. Am J Physiol 246:C204-C215
Hoffman EK, Lambert IH, Simonsen LO (1988) Mechanisms in volume regulation in Erhlich ascites tumor cells. Renal Physiol Biochem 3–5:221–247
Chamberlin ME, Strange K (1989) Anisosmotic cell volume regulation: a comparative view. Am J Physiol 257:C159-C173
Eveloff JL, Warnock DG (1987) Activation of ion transport systems during cell volume regulation. Am J Physiol 252:F1-F10
Fugelli K, Thoroed SM (1986) Taurine transport associated with cell volume regulation in flounder erythrocytes under anisosmotic conditions. J Physiol (Lond) 374:245–261
Hoffmann EK, Lambert IH (1983) Aminoacid transport and cell volume regulation in Ehrlich ascites tumor cells. J Physiol (Lond) 338:613–625
Hoffmann EK (1987) Volume regulation in cultured cells. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 125–180
Grantham JJ, Lowe CM, Dellasega M, Cole B (1977) Effect of hypotonic medium on K and Na content of proximal renal tubules. Am J Physiol 232:F42-F49
Welling PA, Linshaw MA, Sullivan LP (1985) Effect of barium on cell volume regulation in rabbit proximal straight tubules. Am J Physiol 249:F20-F27
Welling PA, Linshaw MA (1988) Importance of anion in hypotonic volume regulation of rabbit proximal straight tubule. Am J Physiol 255:F853-F860
Welling PA, O'Neil RG (1990) Ionic conductive properties of rabbit proximal straight tubule basolateral membrane. Am J Physiol 258:F940-F950
Welling PA, O'Neil RG (1990) Cell swelling activates basolateral Cl and K conductances in rabbit proximal tubule. Am J Physiol 258: F951-F962
Schild L, Aronson PS, Giebisch G (1991) Basolateral transport pathways for K+ and Cl− in rabbit proximal tubule: effects on cell volume. Am J Physiol 260:F101-F109
Knoblauch C, Montrose MH, Murer H (1989) Regulatory volume decrease by cultured renal cells. Am J Physiol 256: C252-C259
Biagi B, Sohtell M, Giebisch G (1981) Intracellular potassium activity in the rabbit proximal straight tubule. Am J Physiol 241: F677-F686
Biagi BA, Sohtell M (1986) pH sensitivity of the basolateral membrane of the rabbit proximal tubule. Am J Physiol 250:F261-F266
Suzuki M, Kawahara K, Ogawa A, Morita T, Kawaguchi Y, Kurihara S, Sakai O (1990) [Ca2+]i rises via G protein during regulatory volume decrease in rabbit proximal tubule cells. Am J Physiol 258: F690-F696
McCarty NA, O'Neil RG (1990) Dihydropyridine-sensitive cell volume regulation in proximal tubule: the calcium window. Am J Physiol 259:F950-F960
Kawahara K, Ogawa A, Suzuki M (1991) Hyposmotic activation of Ca-activated K channels in cultured rabbit kidney proximal tubule cells. Am J Physiol 260:F27-F33
Christensen O (1987) Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature 330:66–68
Sackin H (1987) Stretch-activated potassium channels in renal proximal tubule. Am J Physiol 253:F1253-F1262
Sackin H (1989) A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci USA 86:1731–1735
Sackin H, Palmer LG (1987) Basolateral potassium channels in renal proximal tubule. Am J Physiol 253:F476-F487
Lohr JW (1990) Isovolumetric regulation of renal proximal tubules in hypotonic medium. Renal Physiol Biochem 13:233–240
Rome L, Lechene C, Grantham JJ (1990) Proximal tubule volume regulation in hypo-osmotic media: intracellular K+, Na+, and Cl−. J Am Soc Nephrol 1:211–218
Kirk KL, Schafer JA, Dibona DR (1987) Cell volume regulation in rabbit proximal straight tubule perfused in vitro. Am J Physiol 252: F922-F932
Rome L, Grantham J, Savin V, Lohr J, Lechene C (1989) Proximal tubule volume regulation in hyperosmotic media: intracellular K+, Na+, and Cl−. Am J Physiol 257:C1093–1100
Gilles R (1988) Comparative aspects of cell osmoregulation and volume control. Renal Physiol Biochem 3–5:277–288
Uchida S, Green N, Coon H, Triche T, Mims S, Burg MB (1987) High NaCl induces stable changes in phenotype and karyotype of renal cells in culture. Am J Physiol 253:C230-C242
Bagnasco S, Balaban R, Fales H, Yang YM, Burg M (1986) Predominant osmotically active organic solutes in rat and rabbit medullas. J Biol Chem 261:5872–5877
Bagnasco S, Uchida S, Balaban R, Kador P, Burg MB (1987) Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci USA 84: 1718–1720
Moriyama T, Garcia-Perez A, Burg MB (1990) Factors affecting the ratio of different organic osmolytes in renal medullary cells. Am J Physiol 259:F847-F858
Hebert SC, Sun A (1988) Hypotonic cell volume regulation in mouse medullary thick ascending limb: effects of ADH. Am J Physiol 255:F962-F969
Hebert SC (1986) Hypertonic cell volume regulation in mouse thick limbs. I. ADH dependency and nephron heterogeneity. Am J Physiol 250:C907-C919
Hebert SC (1986) Hypertonic cell volume in mouse thick limbs. II. Na+−H+ and Cl−−HCO3− exchange in basolateral membranes. Am J Physiol 250:C920-C931
Sun A, Hebert SC (1989) Rapid hypertonic cell volume regulation in the perfused inner medullary collecting duct. Kidney Int 36: 831–842
Blumenfeld J, Hebert S, Heilig C, Balschi J, Stromski M, Gullans S (1989) Organic osmolytes in inner medulla of the Brattleboro rat: effects of ADH and dehydration. Am J Physiol 256:F916-F922
Beck F, Dorge A, Rick R, Thurau K (1984) Intra and extracellular elemental concentrations of rat inner papilla in antidiuresis. Kidney Int 25:397–403
Beck FX, Dorge A, Thurau K (1988) Cellular osmoregulation in renal medulla. Renal Physiol Biochem 3–5:174–186
Gilles R (1987) Volume regulation in cells of euryhaline invertebrates. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 205–247
Kleinzeller A (1985) Trimethylamine oxide and the maintenance of volume of dogfish rectal gland cells. J Exp Zool 236:11–17
Goldstein L, Kleinzeller A (1987) Cell volume regulation in lower vertebrates. In: Gilles R, Bolis L, Kleinzeller A (eds) Current topics in membranes and transport, vol 30. Cell volume control: fundamental and comparative aspects in animal cells. Academic Press, San Diego, pp 181–204
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
McDowell ME, Wolf AV, Steer A (1955) Osmotic volumes of distribution: idiogenic changes in osmotic pressure associted with administration of hypertonic solutions. Am J Physiol 180:545–558
Pollock AS, Arieff AI (1980) Abnormalities of cell volume regulation and their functional consequences. Am J Physiol 239: F195-F205
Lohr JW, McReynolds J, Grimaldi T, Acara M (1988) Effect of acute and chronic hypernatremia on myoinositol and sorbitol concentration in rat brain. Life Sci 43:271–276
Law RO (1987) The volume and ionic composition of cells in incubated slices of rat renal cortex, medulla and papilla. Biochem Biophys Acta 931:276–285
Garcia-Perez A, Burg MB (1991) Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol 119: 1–13
Yancey PH, Haner RG, Freudenberger TH (1990) Effects of an aldose reductase inhibitor on organic osmotic effectors in rat renal medulla. Am J Physiol 259:F733-F738
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Linshaw, M.A. Selected aspects of cell volume control in renal cortical and medullary tissue. Pediatr Nephrol 5, 653–665 (1991). https://doi.org/10.1007/BF00856662
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00856662