Abstract
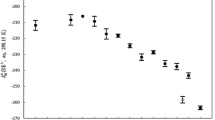
From measurements of the solubility of argon in aqueous solutions of the complexion electrolyte t-[Co(en)2NCSCl]Br at 25°C, the Setschenow parameter for this system is found to be +0.336. Using the scaled-particle theory of the salt effect with this value ofk 5 and the Waddington crystal radius of the Br− ion, 1.98 Å, the radius of the complex cation is calculated to be 3.71 Å. This value is in reasonably good agreement with that estimated from scale models of the t-[Co(en)2-NCSCl]+ cation, about 3.9 Å. It is somewhat larger than the radius calculated from various semiempirical equations relating the molal volume of an ion at infinite dilution to its radius; values of r obtained in this manner range from 3.32 to 3.66 Å.
Similar content being viewed by others
References
W. L. Masterton, Tei Pei Lee, and R. L. Boyington,J. Phys. Chem. 73, 2761 (1969).
S. K. Shoor and K. E. Gubbins,J. Phys. Chem. 73, 498 (1969).
M. Lucas,Bull. Soc. Chem. France, 2994 (1969).
W. L. Masterton and T. P. Lee,J. Phys. Chem. 74, 1776 (1970).
W. L. Masterton, D. Bolocofsky, and T. P. Lee,J. Phys. Chem. 75, 2809 (1971).
J. E. Desnoyers and C. Jolicoeur, inModern Aspects of Electrochemistry, J. O'M. Bockris and B. E. Conway, eds. (Plenum Press, New York, 1969), Vol. 5, Chap. 1.
F. J. Millero,Chem. Rev. 71, 147 (1971).
G. G. Schlessinger,Inorganic Laboratory Preparations (Chemical Publishing Co., Inc., New York, 1962), p. 240.
W.-Y. Wen and J. H. Hung,J. Phys. Chem. 74, 170 (1970); see also Ph.D. thesis of J. H. Hung, Clark University, 1968.
E. W. Washburn, E. R. Smith and M. Frandsen,J. Res. Nat. Bur. Std. 11, 453 (1933).
A. Ben-Naim,J. Phys. Chem. 69, 3245 (1965).
K. A. Becker, G. Grosse, and K. Plieth,Z. Krist. 112, 375 (1959).
P. Mukerjee,J. Phys. Chem. 65, 740 (1961).
E. Glueckauf,Trans. Faraday Soc. 61, 914 (1965).
B. E. Conway, R. E. Verrall, and J. E. Desnoyers,Z. Phys. Chem. (Leipzig) 230, 157 (1965).
Author information
Authors and Affiliations
Additional information
Abstracted, in part, from D.P.'s M.S. thesis, June 1971.
Rights and permissions
About this article
Cite this article
Masterton, W.L., Polizzotti, D. & Welles, H. The solubility of argon in aqueous solutions of a complex-ion electrolyte. J Solution Chem 2, 417–423 (1973). https://doi.org/10.1007/BF00713254
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00713254