Abstract
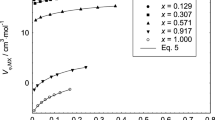
An earlier-developed procedure for calculating the molar volumes of electrolytes Vs0 is used for a comparative analysis. The molar volumes Vs0 are determined for four electrolytes NH4Cl, NaBr, KBr, and KI based on the published data, and the errors of these values are estimated. Comparison of the obtained values of Vs0 and the earlier-found [1] similar values for various cations and anions is performed. It is shown that there is no correlation between Vs0 and the values of the ionic radii.
Similar content being viewed by others
REFERENCES
Glagolenko, Yu.V., Dzekun, E.G., Rovnyi, S.I., Sazhnov, V.K., Ufimtsev, V.P., Broshevitskii, V.S., Laptev, G.A., Osnovin, V.I., Zakharkin, B.S., Smelov, V.S., and Nikipelov, B.V., spent nuclear fuel reprocessing at RT-1 plant, Vopr. Radiats. Bezop., 1997, no. 2, p. 3.
Davis W. and Bruin, H.J., New activity coefficients of 0–100 per cent aqueous nitric acid, J. Inorg. Nucl. Chem., 1964, vol. 26, p. 1069.
Davis, Jr., W., Lawson, P.S., and Mrochek, J., Activities of three components in the system water–nitric acid–uranyl nitrate hexahydrate at 25°, J. Phys. Chem., 1965, vol. 69, p. 1904.
Yu Yang-Xin, Zhang Qing-Yin, and Gao Guang-Hua, Thermodynamics of the system HNO3–UO2(NO3)2–H2O at 298.15 K, J. Radioanal. Nucl. Chem., 2000, vol. 245, no. 3, p. 581.
Ochkin, A.V., Merkushkin, A.O., Nekhaevskii, S.Yu., and Gladilov, D.Yu., Modeling of the activities of uranyl nitrate and nitric acid in mixed solutions, Radiokhimiya, 2018, vol. 60, p. 459.
Zdanovskii, A.B., Lyakhovskaya, E.I., and Shchleimovich, R.E., Handbook of Solubilities of Salt Systems, Leningrad: Goskhimizdat, 1953.
Zdanovskii, A.B., Laws of changes in the properties of mixed solutions, Tr. Solyanoi Lab., Vses. Inst. Galurgii, Akad. Nauk SSSR, 1936, no. 6.
Mikulin, G.I. and Voznesenskaya, I.E., Theory of mixed electrolyte solutions obeying the Zdanovskii rule: 1. Solutions of two salts with a common ion, in Issues of Physical Chemistry of Electrolyte Solutions, Mikulin, G.I. Ed., Leningrad: Khimiya, 1968, p. 304.
Kulov, N.N. and Ochkin, A.V., Method for calculating the density of mixed solutions of strong electrolytes, Theor. Fund. Chem. Technol., 2020, vol. 54, no. 6, p. 1223.
Ochkin, A.V. and Kulov, N.N., Calculation of seawater density, Theor. Fund. Chem. Technol., 2022, vol. 55 (in press).
CRC Handbook of Chemistry and Physics, Lide, D.R., Ed., 2005, 86th ed.
Voznesenskaya, I.E. and Mikulin, G.I., Tables of water activity in solutions of strong electrolytes at 25°C, in Issues of Physical Chemistry of Electrolyte Solutions, Mikulin, G.I. Ed., Leningrad: Khimiya, 1968, p. 361.
Funding
This work was financially supported by the Ministry of Education and Science of the Russian Federation within a state task by project FSSM-2020-0004.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Ochkin, A.V., Kulov, N.N. Comparison of the Molar Volumes of Some Electrolytes. Theor Found Chem Eng 56, 644–649 (2022). https://doi.org/10.1134/S0040579522050311
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579522050311