Summary
In order to evaluate the role of glucose-phosphate isomerase (GPI) inFundulus heteroclitus, the isozymes and allozymes were purified and some of their physical and kinetic properties determined.
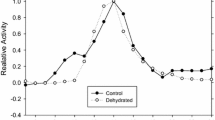
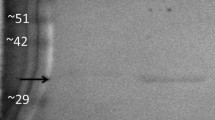
Isozymes were purified from both liver (GPI-B) and muscle (GPI-A) tissue (Tables 1, 2). Gel filtration of the native enzyme and SDS-polyacrylamide gel electrophoresis indicated that all forms are dimers of approximately 110,000 Daltons (Figs. 4, 5). Although thermal stability studies revealed no differences between the allozymes, the isozymes were clearly different (Figs. 6, 7). Kinetic analysis showed further differences between isozymes inK m for substrate andK I for 6-phosphogluconate (Figs. 8, 9; Table 3). No significant differences were found between the allozymes of the B-locus under the conditions employed in this study.
Based on the tissue specificities and the functional differences between isozymes, we propose a possible regulatory role for GPI-B inF. heteroclitus. The sensitivity of this isozyme to 6-phosphogluconate inhibition may allow GPI-B to act as a regulatory enzyme in the partitioning of carbon flow between glycolysis and the hexose monophosphate shunt.
Similar content being viewed by others
Abbreviations
- βme :
-
β-mercaptoethanol
- F6P :
-
fructose-6-phosphate
- G1P :
-
glucose-1-phosphate
- G6P :
-
glucose-6-phosphate
- G6Pase :
-
glucose-6-phosphatase
- G6PDH :
-
glucose-6-phosphate dehydrogenase
- GPI :
-
glucosephosphate isomerase
- HK :
-
hexokinase
- HMP :
-
hexose monophosphate shunt
- 6PG :
-
6-phosphogluconate
- PGM :
-
phosphoglucomutase
References
Ackers, G.K.: A new calibration procedure for gel filtration columns. J. Biol. Chem.242, 3237–3238 (1967)
Avise, J.C., Kitto, G.B.: Phosphoglucose isomerase gene duplication in the bony fishes. An evolutionary history. Biochem. Genet.8, 113–132 (1973)
Avise, J.C., Selander, R.K.: Evolutionary genetics of cave dwelling fishes of the genusAstyamax. Evolution26, 1–19 (1972)
Baich, A., Wolfe, R.G., Reithel, F.J.: The enzymes of mammary gland. I. Isolation of phosphoglucose isomerase. J. Biol. Chem.235, 3130–3133 (1960)
Carter, N.D., Parr, C.W.: Isoenzymes of phosphoglucose isomerase in mice. Nature216, 511 (1967)
Cleland, W.W.: The statistical analysis of enzyme kinetic data. Adv. Enzymol.29, 1–32 (1967)
Cornish-Bowden, A.: Principles of enzyme kinetics. London: Butterworths 1976
Dando, P.R.: Distribution of multiple glucosephosphate isomerases in teleostean fishes. Comp. Biochem. Physiol.47B, 663–679 (1974)
Day, T., Hiller, P., Clarke, B.: The relative quantities and catalytic activities of enzymes produced by alleles at the alcohol dehydrogenase locus inDrosophila melanogaster. Biochem. Genet.11, 155–165 (1974a)
Day, T., Needham, L.: Properties of alcohol dehydrogenase isozymes in a strain ofDrosophila melanogaster homozygous for the Adh-slow allele. Biochem. Genet.11, 167–175 (1974b)
DeLorenzo, R.J., Ruddle, F.H.: Genetic control of two electrophoretic variants of glucosephosphate isomerase in the mouse (Mus musculus). Biochem. Genet.3, 151–162 (1969)
Detter, J.C., Ways, P.O., Giblett, E.R., Baughman, M.A., Hopkinson, D.A., Povey, S., Harris, H.: Inherited variations in human phosphohexose isomerase. Ann. Hum. Genet.31, 329–338 (1968)
Dixon, N., Webb, E.C.: Enzymes. London, N.Y., Toronto: Longmans, Green and Co. 1958
Dyson, J.E.D., Noltman, E.A.: The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. J. Biol. Chem.243, 1401–1414 (1968)
Fairbanks, G., Steck, T., Wallach, D.: Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochem.10, 2606–2617 (1971)
Fjellstedt, T., Schlesselman, J.: A simple statistical method for use in kinetic analysis based on Lineweaver-Burke plots. Anal. Biochem.80, 224–238 (1977)
Fried, G.H., Schriebman, M.P., Kallman, K.D.: Enzymatic activity in tissue of teleosts. Comp. Biochem. Physiol.28, 771–776 (1969)
Gill, P., Itzhaki, R.: A micro-biuret method for estimating proteins. Anal. Biochem.9, 401–410 (1964)
Gracy, R.W.: Nature of the multiple forms of glucose phosphate and triose phosphate isomerases. Proc. 3rd Internatl. Conf. Isozymes, Vol. 1. Markert, C. (ed.), pp. 471–487. New York: Academic Press 1974
Greenbaum, A.L., Gumaa, K.A., McLain, P.: The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch. Biochem. Biophys.143, 617–663 (1971)
Johnson, A.G., Utter, F.M., Hodgins, H.O.: Estimate of genetic polymorphism and heterozygosity in three species of rockfish (genusSebastes). Comp. Biochem. Physiol.44B, 397–406 (1973)
Johnson, G.: Enzyme polymorphism and metabolism. Science184, 28–37 (1974)
Jungermann, K., Sasse, D.: Heterogeneity of liver parenchymal cells. Trends Biochem. Sci.3, 198–202 (1978)
Kather, H., Rivera, M., Brand, K.: Interrelationship and control of glucose metabolism and lipogenesis in isolated fat cells: Control of pentose phosphate-cycle activity by cellular requirement for reduced nicotinamide adenine dinucleotide phosphate. Biochem. J.128, 1097–1102 (1972)
Kempe, T.D., Nakagawa, Y., Noltman, E.: Physical and chemical properties of yeast phosphoglucose isomerase isoenzymes. J. Biol. Chem.249, 4617–4624 (1974a)
Kempe, T.D., Gee, D., Hathaway, G., Noltman, E.: Subunit and peptide compositions of yeast phosphoglucose isomerase isoenzymes. J. Biol. Chem.249, 4625–4633 (1974b)
Laemmli, U.: Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature227, 680–685 (1970)
Landau, B., Katz, J.: A quantitative estimation of the pathways of glucose metabolism in rat adipose tissuein vitro. J. Biol. Chem.239, 697–704 (1964)
Markert, C. (ed.). The isozymes. New York: Academic Press 1975
Mathers, N.F., Wilkins, N.P., Walne, P.R.: Phosphoglucose isomerase and esterase phenotypes inCrassostrea angulata andC. gigas. Biochem. Sys. Ecol.2, 93–96 (1974)
Merrit, R.B.: Geographic distribution and enzymatic properties of lactate dehydrogenase allozymes of the fathead minnow,Pimephalis promelas. Am. Nat.106, 173–184 (1972)
Mitton, J., Koehn, R.: Genetic organization and adaptive response of allozymes to ecological variables inFundulus heteroclitus. Genetics79, 97–111 (1975)
Mo, Y., Young, C., Gracy, R., Carter, N., Dando, P.: Isolation and characterization of tissue-specific isozymes of glucose-phosphate isomerase from catfish and conger. J. Biol. Chem.250, 6747–6755 (1975)
Noltman, E.A.: Glucose-6-phosphate isomerase. In: The enzymes, 3rd ed., Vol. VI. Boyer, P.D. (ed.), pp. 272–301. New York: Academic Press 1972
Place, A.R., Powers, D.A.: Liver LDH allozymes ofFundulus heteroclitus: A selective adaptation or genetic drift? Fed. Proc.36, 738 (1977)
Place, A.R., Powers, D.A.: Genetic bases for protein polymorphism inFundulus heteroclitus (L.). I. Lactate dehydrogenase (Ldh-B), malate dehydrogenase (Mdh-A), glucosephosphate isomerase (Gpi-B) and phosphoglucomutase (Pgm-A). Biochem. Genet.16, 577–591 (1978)
Place, A.R., Powers, D.A.: Genetic variation and relative catalytic efficiencies: Lactate dehydrogenase B allozymes ofFundulus heteroclitus. Proc. Natl. Acad. Sci.76, 2354–2358 (1979)
Powers, D.A., Place, A.R.: Biochemical genetics ofFundulus heteroclitus (L.) I. Temporal and spatial variation in gene frequencies of Ldh-B, Mdh-A, Gpi-B, and Pgm-A. Biochem. Genet.16, 593–607 (1978)
Powers, D.A., Powers, D.: Predicting gene frequencies in natural populations: A testable hypothesis. In: The isozymes, Vol. IV. Markert, C. (ed.), pp. 63–84. San Francisco: Academic Press 1975
Saison, R.: Serum and red cell enzyme systems in pigs. Proc. XIth Eur. Conf. Animal Blood Groups Biochem. Polymorphisms. pp. 321–330. Warsaw: Polish Scientific Publishers 1968
Selander, R.K., Yang, S.R., Lewontin, R.C., Johnson, W.E.: Genetic variation in the horseshoe crab (Limulus polyphemus), a phylogenetic “relic”. Evolution24, 402–414 (1970)
Shaw, C., Prasad, R.: Starch gel electrophoresis of enzymes —a compilation of recipes. Biochem. Genet.4, 297–320 (1970)
Tilley, B.E., Gracy, R.W., Welch, S.G.: A point mutation increasing the stability of human phosphoglucose isomerase. J. Biol. Chem.249, 4571–4579 (1974)
Welch, S.G.: Qualitative and quantitative variants of human phosphoglucose isomerase. Human Hered.21, 467–477 (1971)
Welch, S., Fitch, L., Parr, C.: A variant of rabbit phosphoglucose isomerase. Biochem. J.117, 525–531 (1970)
Wilkins, N.P.: Biochemical genetics of the Atlantic Salmon (Salmo salar L.) II. The significance of recent studies and their application in population identification. J. Fish Biol.4, 505–517 (1972)
Wilkinson, F.H.: Statistical estimations in enzyme kinetics. Biochem. J.80, 324–332 (1961)
Yndgaard, C.F.: Genetically determined electrophoretic variants of phosphoglucose isomerase and 6-phosphogluconate dehydrogenase inZoarces viviparus L. Hereditas71, 151–154 (1972)
Author information
Authors and Affiliations
Additional information
Supported in part by: NSF grants DEB-76-19877 to D.A.P. and PCM 77-16838 to B.D.S., NIH Biomedical grant 5-50-7RR07-041 and a grant from the National Geographic Society. G.D.S. and R.V.B. are NIH trainees supported by a training grant (No. HD00139) to the Department of Biology, The Johns Hopkins University. This is contribution No. 1052 from the Department of Biology
Rights and permissions
About this article
Cite this article
Palumbi, S.R., Sidell, B.D., Van Beneden, R. et al. Glucosephosphate isomerase (GPI) of the teleostFundulus heteroclitus (linnaeus): Isozymes, allozymes and their physiological roles. J Comp Physiol B 138, 49–57 (1980). https://doi.org/10.1007/BF00688735
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00688735